Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.17 n.1 Pretoria 2023
http://dx.doi.org/10.7196/SAJCH.2023.v17i1.1939
RESEARCH
Outcome of infants with necrotising enterocolitis at Charlotte Maxeke Johannesburg Academic Hospital, South Africa
M SelseI; R T SaggersII; E HentzIII, IV; A ElfvinIII, IV; D E BallotV
IMD; Department of Paediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
IIMB BCh, FC Paed (SA), MMed (Paed); Department of Paediatrics and Child Health, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMD, PhD; Department of Paediatrics, Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
IVMD, PhD; Region Vastra Götaland, Department of Paediatrics, The Queen Silvia Children's Hospital, Sahlgrenska University Hospital, Gothenburg, Sweden
VMB BCh, FC Paed (SA), PhD; School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Necrotising enterocolitis (NEC) is an inflammatory disease almost exclusively affecting preterm infants. Previous research has presented a higher mortality rate in infants requiring surgical treatment compared with infants receiving medical treatment. However, the knowledge of mortality and morbidity of the disease in low- and middle-income countries is still limited
OBJECTIVES: To review infants with NEC admitted to the neonatal unit at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH), determine a potential difference in mortality between medically and surgically treated infants, and to identify characteristics and factors associated with mortality among these infants
METHODS: This retrospective study described infants with NEC born between 1 January 2016 and 31 December 2018 who were admitted to the neonatal unit. The characteristics and survival of these infants were compared using univariate and multivariate analyses
RESULTS: During the study period, 5 061 infants were admitted to the neonatal unit, of which 218 infants were diagnosed with NEC. The period prevalence of NEC was 4.3% among all neonatal infants and 11.0% among very-low-birthweight (VLBW) infants. Mortality was significantly higher among surgically treated infants with NEC compared with medically treated infants (p=0.025, odds ratio 1.888 (95% confidence interval 1.082 - 3.296)). Late-onset sepsis was significantly more common among VLBW infants with NEC (71.3%) compared with VLBW infants without NEC (27.1%). Among infants with late-onset sepsis, Gram-negative bacteria, multidrug-resistant bacteria and fungal sepsis was significantly more common in the group of infants with NEC
CONCLUSIONS: Infants with NEC treated surgically at CMJAH have an increased risk of dying compared with those receiving medical treatment, likely due to the severity of disease. Furthermore, this study emphasised the burden of sepsis among infants with NEC and may contribute to a better knowledge of NEC in South Africa
Keywords: necrotising enterocolitis; mortality; prematurity; late-onset sepsis.
During the neonatal period (defined as the first 28 days of life) infants are the most vunerable. Liu et al.[1] estimated that in 2015, 45.1% of all global deaths of children under 5 years old occurred during the neonatal period, with preterm birth being one of the most common causes of death among neonates. Necrotising enterocolitis (NEC) is a severe inflammatory disease with a multifactorial pathogenesis, almost exclusively affecting preterm infants.[2] NEC usually develops during the first weeks of life, after the initiation of enteral feeding.[3] The first clinical signs of NEC are often nonspecific, such as poor feeding, abdominal distension, lethargy and bloody stools.[2] The condition can progress rapidly to a lethal stage of the disease, resulting in a high mortality rate.[2 The disease is classified into three stages by the modified Bell staging system.[4] Depending on the severity of NEC, the treatment consists of medical or surgical intervention. The medical treatment, which is often sufficient in NEC stage 1 and 2, includes broad-spectrum antibiotics and total parenteral nutrition with bowel rest, along with clinical monitoring to evaluate the course of the disease.[2] Surgical treatment is needed when there is clinical deterioration despite adequate medical management, or when there are signs of intestinal perforation.[5] The surgical interventions are either peritoneal drainage or laparotomy with bowel resection.[2] At Charlotte Maxeke Johannesburg Academic Hospital (CMJAH), peritoneal drainage is considered a temporary solution, performed when infants are too unstable to undergo laparotomy. The two most common interventions during a laparotomy are primary anastomosis or enterostomy.[6]
In a review from several high-income countries,[7] the incidence of NEC varies from 2% to 7% in infants born before 32 gestational weeks. An American study published by Hull et al.[8] showed a significantly higher mortality among infants with NEC requiring surgical treatment, compared with infants receiving medical treatment. Other studies from high-income countries have presented similar differences in mortality.[9] Hypothetically, there would be a similar difference in mortality between medically and surgically managed NEC in a low- and middle-income country.
There is limited research from low- and middle-income countries focusing on NEC. In a study from Cape Town,[10] the overall mortality in infants with NEC admitted to the neonatal intensive care unit was 54%. At CMJAH, Ballot et al.[11] demonstrated a period prevalence of NEC in 2016 of 9.5% among infants with birthweight of >1 500 grams and 24.7% in very-low-birthweight (VLBW) infants (birthweight <1 500 g). The mortality rate among VLBW infants with NEC was 62.5% (95% confidence interval (CI) 51.3 - 73.7).[11] However, this study only included infants admitted to the combined paediatric and neonatal intensive care unit (PNICU). Therefore, more knowledge would contribute to a better comprehension of the local burden of disease caused by NEC.
Objectives
The present study aimed to determine whether there was a difference in mortality between medically and surgically treated infants with NEC and to explore the extent of this potential difference. Secondary objectives were to see if there was a difference in mortality between infants with NEC receiving different interventions during a surgical laparotomy, and if there was a difference in mortality between infants receiving surgical and medical treatment within different birthweight groups. The present study compared characteristics between the infants with NEC and those without NEC. Additionally, an analysis to determine factors associated with increased mortality within the NEC group was performed.
Methods
Study design
This retrospective study used data collected at CMJAH - a large surgery referral hospital in Johannesburg, South Africa (SA) with paediatric surgery capabilites. The neonatal unit at CMJAH consists of 95 beds, of which 15 beds are available in the combined PNICU. Patient data have been collected from the neonatal unit, including infants admitted to the PNICU, since 2013, as a part of a quality improvement programme of neonatal care.
Data included demographics, maternal and infant information and medical information during the hospitalisation. This information was collected from patient records at discharge, transcribed to control sheets and later entered onto the Research Electronic Data Capture (REDCap) database. The database was hosted by the University of the Witwatersrand. Data were verified multiple times during the data collection process to ensure accuracy.
Study population
The participants in this study were infants born between the 1 January 2016 and 31 December 2018 who were admitted to the neonatal unit. Deidentified data from all infants admitted to the neonatal units at CMJAH during this time were extracted from the REDCap database. Infants with missing information on critical data were excluded from the analyses.
Study procedure
Variables considered relevant to the objectives were extracted from the database. The primary outcome was mortality before discharge. Infants who were either discharged home or transferred to another ward or hospital were considered to have survived. Data on confirmed NEC diagnosis included stage 2 and 3 only, diagnosed by clinical signs and characteristic radiographical findings according to the modified Bell's staging criteria.[4] Birthweight was used as a measure of prematurity instead of gestational age, since birthweight was considered more reliable in this setting.
The different surgical interventions were peritoneal drainage, laparotomy with bowel resection and primary anastomosis and laparotomy with bowel resection and enterostomy. For a few of the infants who had undergone laparotomy, information on intervention during laparotomy was missing. These infants were excluded from the secondary analysis.
Sepsis was defined as a positive blood culture of an organism deemed to be significant. An onset within 72 hours of birth was classified as early-onset sepsis, while onset after 72 hours of birth was classified as late-onset sepsis.'121 All pathogens, including coagulase-negative staphylococci, were considered significant in late-onset sepsis, since blood cultures were only collected if infants had symptoms or clinical signs of an infection.
Birthweight was grouped into birthweight <1 000 g, birthweight of 1 000 - 1 499 g and birthweight >1 500 g. In the comparison of characteristics and the analysis of factors associated with mortality, birthweight was included as a continuous variable.
Data analysis
All data analyses were performed using IBM SPSS version 26 (IBM Corp., USA). Descriptive statistics for continuous data were presented with mean and standard deviation (SD) for normally distributed data and median and interquartile range (IQR) for skewed data. Categorical data were described with frequencies (n) and percentages (%). Missing cases were excluded from the analyses. Univariate analysis was performed to compare infants with and without NEC, and infants who died from NEC compared with infants who survived. Chi-square tests and calculation of odds ratio were performed on the categorical variables. Odds ratio was calculated for the analyses with a significant Chi-square test. For the continuous variables, the Mann-Whitney U-test was used when data were skewed, and for the normally distributed variables, independent sample t-tests were performed. A p-value of <0.05 was considered significant and 95% CIs were presented. Variables with a p-value <0.1 on univariate analysis were entered into a binary logistic regression model to determine the most significant association with NEC.
Ethics
This project was integrated into an ethical clearance approved by the Human Research Ethics Committee of the University of the Witwatersrand (ref. no. M160338).
Results
A total of 5 061 infants were admitted to the neonatal unit at CMJAH during the study period. The patient selection for the analyses is shown in Fig. 1. A diagnosis of NEC stage 2 or 3 was made in 218 infants, of which 217 were included in the primary analysis. The period prevalence of NEC among all infants was 4.3%. There were 1 444 infants with VLBW; in this group, the period prevalence of NEC was 11.0%. The period prevalence of NEC for inborn VLBW infants was 6.9%.
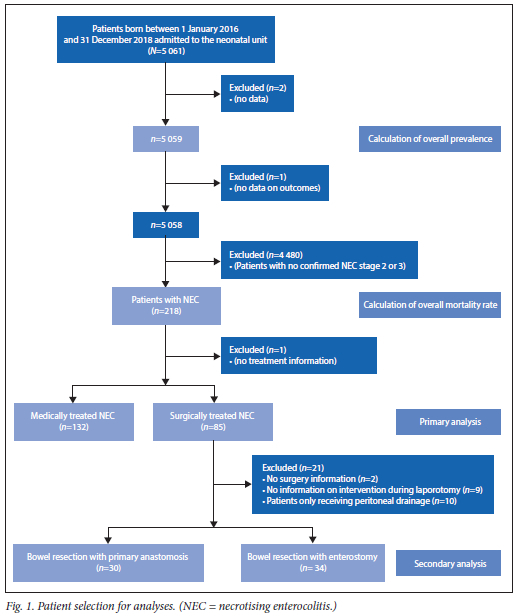
The median (IQR) birthweight of all infants admitted to a neonatal unit was 2 100 (1 400 - 3 010) grams, with a median (IQR) gestational age of 35 (31 - 39) weeks; 54.1% of all infants were male (n=2 735). The majority were born at CMJAH (n=3 952; 78.1%). The mean (SD) maternal age was 28.9 (6.4) years.
A comparison ofcharacteristics between the infants with a diagnosis of NEC versus the other infants was performed. Subsequently, the variables significantly different between the groups were analysed within the VLBW population. Birthweight and significant variables are shown in Table 1. Antenatal corticosteroid use was not significant within the VLBW group.
Variables with p<0.1 were entered into binary logistic regression to determine which were most significantly associated with NEC. The results are shown in Table 2. Out of the 218 infants diagnosed with NEC, 87 infants died, resulting in an overall mortality rate of 39.9%. In the subgroup of VLBW infants with NEC, 43.4% died. In the group with medically treated NEC, the mortality rate was 34.1%, and in the group requiring surgical treatment, the mortality rate was 49.4%. There was a statistically significant difference in mortality rate between these two groups (p=0.025). The odds ratio of dying from NEC requiring surgery compared with conservatively treated NEC was 1.89 (95% CI 1.08 - 3.30). There was no statistical significance between the two treatment groups when a comparison within the three different birthweight groups was performed. Mortality within the group requiring peritoneal drainage was significantly higher than within the group undergoing laparotomy (90.0% and 42.5% respectively, p=0.006). A third (n=10/30) of the infants received an anastomosis died. Among the infants received an enterostomy, 47.1% (n=16/34) died. No significant difference in mortality between these two groups (primary anastomosis v. enterostomy) was found (p=0.265).
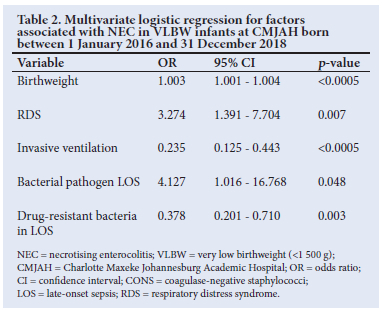
The variables significantly associated with mortality within the NEC group are listed in Table 3, along with HIV exposure, which was more common in the survival group.
Discussion
The present study showed that infants with NEC requiring surgical treatment had a significantly higher mortality, compared with medically treated infants. The findings from the primary analysis are consistent with those of Hull et al.,[8] suggesting a significantly higher mortality rate in surgically treated infants. These results are likely to be related to previous implications that infants requiring surgical treatment are more critically ill than medically treated infants.[2,13] However, the mortality rates in our study of 34.1% and 49.4% in medically and surgically treated infants, respectively, were higher than previously reported mortality rates.[8] A possible explanation for this is that there could be differences in the neonatal care between high- and middle-income countries affecting the mortality rates for NEC. This theory is supported by the global variation in the incidence and complications of prematurity, as well as the variation in neonatal mortality and morbidity.[14,15] In contrast to the present study, Hull et al.[8] studied VLBW infants only, which is worth taking into consideration when comparing the results. Nonetheless, the overall mortality rate for VLBW infants in our study was 43.4%, which was higher as well.
In the study from the PNICU at CMJAH by Ballot et al.,[11] the mortality rate among VLBW infants with NEC was higher than in our study, as well as the mortality rates presented in the study by Satardien et al.[10] among infants admitted to a neonatal intensive care unit. However, infants admitted to an intensive care unit are presumably more sick and more likely to require surgery, which might have an increasing impact on the mortality rate. Therefore, a comparison with these findings must be interpreted with caution. Additionally, many infants with a confirmed diagnosis of NEC will be admitted to an intensive care unit due to the often severe nature of NEC, which might explain the higher period prevalence of NEC in previous studies. [10,11] In the article by Satardien et al.[10], surgical treatment was performed more often and earlier in survivors as compared with non-survivors. However, >50% of the infants who did not undergo surgery had care redirected to palliation, which might explain these conflicting results.[10]
Our study presented a slightly higher period prevalence than previously reported results by Hull et al.,[8] which reported 9% of the VLBW infants developed NEC. This may be explained by the fact that CMJAH is a referral hospital to where infants requiring more specialised care are referred, reflected by the lower prevalence for inborn infants.
The high mortality within the patient group requiring peritoneal drainage was in line with previous research, and not very surprising owing to the temporary usage of the surgical method in infants too unstable to undergo laparotomy immediately. [8] Consistent with previous literature, no significant difference was seen when comparing mortality rates of infants with the two surgical interventions (primary anastomosis and enterostomy) in this study. [16] Owing to the inflammatory nature of the disease, poor healing with leakage is a possible complication after creating an anastomosis, which might explain why an enterostomy is a more common surgical intervention for NEC in many countries. However, enterostomies require advanced postoperative care which, in a country with less resources, could potentially lead to even more severe complications. This was discussed in a previous study from Johannesburg by Banieghbal et al.[17] and could be a possible explanation why primary anastomosis is still a common intervention during laparotomy for NEC at CMJAH.
Through the comparison of characteristics, it is shown that infants with NEC are more acutely ill, illustrating the severity of the disease. Prolonged oxygen use and cranial ultrasound abnormalities were more common among infants with NEC, possibly a reflection of the overall degree of illness. It is known that sepsis is common among infants with NEC, which the results in this comparison emphasise. The qualities of late-onset sepsis in this study are also consistent with results from recent studies; a previous study has shown a high prevalence of Gram-negative bloodstream infections among infants with NEC.[18] Another study[19] implicated a worse outcome in NEC-infants with Gram-negative bacteraemia. The significantly higher prevalence of fungal sepsis and drug-resistant bacteria among infants with NEC may partly be explained by antibiotic usage in this group, but more research is desirable to determine a correlation. Patent ductus arteriosus was more common in the group with NEC, supporting the previous associations between this factor and NEC.[20,21] Additionally, blood transfusions were more common among infants with NEC. This was seen in previous research. However, larger studies have failed to demonstrate this association.[21,22] There is ongoing discussion about whether anaemia is a potential risk factor, resulting in an increase of blood transfusions among infants with NEC.[22] This study also found that lower birthweight was associated with mortality among infants with NEC, consistent with previous literature.[23] Invasive ventilation is needed in more severely ill infants, which makes the observed association with mortality fairly expected. A curious finding in this study was that HIV exposure was more common in the survival group. In future studies, it would be of interest to explore if the findings are consistent in a larger population.
Study limitations
Although data were verified repeatedly, there were some missing data. Additionally, since the study was retrospective, some information was not available. For example, information on the timing of NEC diagnosis and time of onset of infections was missing, making it impossible to draw conclusions regarding risk factors for NEC. It is also difficult to evaluate if any deaths among infants with NEC were due to a condition other than NEC, which would be of value when examining the mortality rates. Further information on feeding practices before onset of NEC was unavailable, as well as maternal information on preeclampsia and chorioamnionitis, factors considered in the literature to impact the development of NEC. The limited sample size in some of the analyses might conceal a significant difference, and larger studies looking at mortality of NEC would therefore be of value.
Conclusion
The present retrospective study has shown a higher mortality rate in infants with NEC requiring surgical treatment, compared with medically treated infants. This is likely due to the fact that infants with more severe NEC are more likely to require surgical rather than medical management. Furthermore, this study has highlighted factors and characteristics associated with NEC and an increased mortality, which could be useful in order to gain a better knowledge of the burden of NEC at CMJAH and to evaluate interventions and improvements of the neonatal care. Additional research from low-and middle-income countries would help improve the knowledge of the disease in these countries. Future studies including several hospitals with similar databases in SA would contribute to mortality rates more applicable throughout the country, with a depiction of differences within the country. Additionally, the present study did not look at socio-economic factors associated with mortality of NEC. This could be of interest for future research.
Declaration. None.
Acknowledgements. The authors gratefully thank the staff at CMJAH working with the database and contributing with valuable assistance on data management.
Author contributions. MS conceived the study design, collected data, carried out the univariate analyses and wrote the manuscript. RTS conceived the study design, carried out the multivariate analysis, supervised the study, reviewed and revised the manuscript and approved the final manuscript. EH, AE and DEB conceived the study design, supervised the study, reviewed and revised the manuscript and approved the final manuscript.
Funding. This work was supported by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF agreement (grant no. ALFGBG-117661).
Conflicts of interest. None.
References
1. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000 - 15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016;388(10063):3027-3035 https://doi.org/10.1016/S0140-6736(16)31593-8 [ Links ]
2. Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364(3):255-264. https://doi.org/10.1056/NEJMra1005408 [ Links ]
3. Yee WH, Soraisham AS, Shah VS, Aziz K, Yoon W, Lee SK. Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics 2012;129(2):e298-304. https://doi.org/10.1542/peds.2011-2022 [ Links ]
4. Walsh MC, Kliegman RM. Necrotizing enterocolitis: Treatment based on staging criteria. Pediatr Clin North Am 1986;33(1):179-201. https://doi.org/10.1016/s0031-3955(16)34975-6 [ Links ]
5. Frost BL, Modi BP, Jaksic T, Caplan MS. New medical and surgical insights into neonatal necrotizing enterocolitis: A review. JAMA Pediatr 2017;171(1):83-88. https://doi.org/10.1001/jamapediatrics.2016.2708 [ Links ]
6. Thakkar HS, Lakhoo K. The surgical management of necrotising enterocolitis (NEC). Early Hum Dev 2016;97:25-28. https://doi.org/10.1016/j.earlhumdev.2016.03.002 [ Links ]
7. Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high-income countries: A systematic review. Arch Dis Child Fetal Neonatal Ed 2018;103(2):F182-F189. https://doi.org/10.1136/archdischild-2017-313880 [ Links ]
8. Hull MA, Fisher JG, Gutierrez IM, et al. Mortality and management of surgical necrotizing enterocolitis in very low birth weight neonates: A prospective cohort study. J Am Coll Surg 2014;218(6):1148-1155. https://doi.org/10.1016/j.jamcollsurg.2013.11.015 [ Links ]
9. Jones IH, Hall NJ. Contemporary outcomes for infants with necrotizing enterocolitis - a systematic review. J Pediatr 2020;220:86-92. https://doi.org/10.1016/j.jpeds.2019.11.011 [ Links ]
10. Satardien M, Van Wyk L, Sidler D, Van Zyl JI. Outcomes of neonates requiring neonatal intensive care admission for necrotizing enterocolitis in a resource-restricted hospital in Cape Town, South Africa. J Trop Pediatr 2021;67(1):1-10. https://doi.org/10.1093/tropej/fmaa130 [ Links ]
11. Ballot DE, Davies VA, Cooper PA, Chirwa T, Argent A, Mer M. Retrospective cross-sectional review of survival rates in critically ill children admitted to a combined paediatric/neonatal intensive care unit in Johannesburg, South Africa, 2013 - 2015. BMJ Open 2016;6(6):e010850. https://doi.org/10.1136/bmjopen-2015-010850 [ Links ]
12. Shane AL, Sánchez PJ, Stoll BJ. Neonatal sepsis. Lancet 2017;390(10104):1770-1780. https://doi.org/10.1016/s0140-6736(17)31002-4 [ Links ]
13. Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, Clark RH. Necrotizing enterocolitis among neonates in the United States. J Perinatol 2003;23(4):278-285. https://doi.org/10.1038/sj.jp.7210892 [ Links ]
14. World Health Organization. Neonatal Mortality Rate, Year: 2018. Geneva: WHO, 2018. http://apps.who.int/gho/data/node.sdg.3-2-viz-3?lang=en (accessed 27 March 2020). [ Links ]
15. Platt MJ. Outcomes in preterm infants. Public Health 2014;128(5):399-403. https://doi.org/10.1016/j.puhe.2014.03.010 [ Links ]
16. ingh M, Owen A, Gull S, Morabito A, Bianchi A. Surgery for intestinal perforation Sin preterm neonates: Anastomosis vs stoma. J Pediatr Surg 2006;41(4):725-729. https://doi.org/10.1016/j.jpedsurg.2005.12.017 [ Links ]
17. Banieghbal B, Davies MR. Damage control laparotomy for generalised necrotizing enterocolitis. World J Surg 2004;28(2):183-186. https://doi.org/10.1007/s00268-003-7155-9 [ Links ]
18. Bizzarro MJ, Ehrenkranz RA, Gallagher PG. Concurrent bloodstream infections in infants with necrotizing enterocolitis. J Pediatr 2014;164(1):61-66. https://doi.org/10.1016/j.jpeds.2013.09.020 [ Links ]
19. Elfvin A, Dinsdale E, Wales PW, Moore AM. Low birthweight, gestational age, need for surgical intervention and Gram-negative bacteraemia predict intestinal failure following necrotising enterocolitis. Acta Paediatr 2015;104(8):771-776. https://doi.org/10.1111/apa.12997 [ Links ]
20. Rõjâs S, Borg H, Edenholm M, Sandberg K, Elfvin A. Abdominal pathology requiring laparotomy in very preterm infants is associated with need for surgical closure of patent ductus arteriosus. J Pediatr Surg 2011;46(10):1898-1902. https://doi.org/10.1016/j.jpedsurg.2011.06.028 [ Links ]
21. Rose AT, Patel RM. A critical analysis of risk factors for necrotizing enterocolitis. Semin Fetal Neonatal Med 2018;23(6):374-379. https://doi.org/10.1016/j.siny.2018.07.005 [ Links ]
22. Hay S, Zupancic JA, Flannery DD, Kirpalani H, Dukhovny D. Should we believe in transfusion-associated enterocolitis? Applying a GRADE to the literature. Semin Perinatol 2017;41(1):80-91. https://doi.org/10.1053/j.semperi.2016.09.021 [ Links ]
23. Fitzgibbons SC, Ching Y, Yu D, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg 2009;44(6):1072-1075. https://doi.org/10.1016/j.jpedsurg.2009.02.013 [ Links ]
 Correspondence:
Correspondence:
M Selse
moa.selse@outlook.com
Accepted 4 January 2022














