Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.16 no.4 Pretoria Dez. 2022
http://dx.doi.org/10.7196/SAJCH.2022.v16i4.1862
RESEARCH
Children and adolescents with diabetes at Tygerberg Hospital - at risk of cardiovascular complications?
L N DookhonyI; C J LombardII; E W ZöllnerIII
IMMed (Paeds); Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa; and SSRN Hospital, Pamplemousses, Republic of Mauritius
IIMSc, PhD; Biostatistics Unit, South African Medical Research Council, Division of Biostatistics; and Department of Global Health, University of Stellenbosch, Cape Town, South Africa
IIIMMed, PhD; Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
ABSTRACT
BACKGROUND. In South Africa, the prevalence of dyslipidaemia and hypertension (HT) in paediatric diabetes patients is unknown.
OBJECTIVES. To determine the prevalence of dyslipidaemia and HT in paediatric diabetic patients seen at Tygerberg Hospital (TBH) and establish whether either is associated with body mass index (BMI), glycosylated haemoglobin (HbA1c) or duration of diabetes. Further, to determine whether the prevalence differs between two specified periods.
METHODS. A retrospective study of 154 diabetic patients, aged 1 - 19 years, seen at TBH between 2007 and 2017, was undertaken. The following data were recorded: age; sex; duration of disease (time since diagnosis); height; weight; blood pressure; HbA1c; high-density lipoprotein cholesterol (HDL-C); triglycerides (TG); and low-density lipoprotein cholesterol (LDL-C).
RESULTS. More than half of the patients (57.8%; n=89/154; 95% confidence interval (CI) 51.7 - 65.0) had dyslipidaemia, 16.3% (n=24/147) had low HDL-C levels, 53.8% (n=78/145) had high LDL-C levels and 14.9% (n=22/148) had raised TG levels. Nearly half of the patients (48.7%; n=75/154; 95% CI 41.6 - 55.1) were hypertensive and 93.5% (n=144/154) were poorly controlled (HbA1c >7.5%). Dyslipidaemia was not associated with HT or BMI percentile and its prevalence did not change between the two specified periods. Prevalence of dyslipidaemia and HT was not associated with duration of diabetes. About one-third (30.8% (n=4/13); 95% CI 11.9 - 59.3) of the pre-adolescents and 60.3% (n=85/141; 95% CI 51.9 - 68.1) of the adolescents had dyslipidaemia (p=0.04). Dyslipidaemia was diagnosed in 62.6% (n=82/131) of adolescents with poorly controlled diabetes (p=0.04) and in 71.7% (95% CI 59.0 - 81.7) of patients >16 years of age (p=0.005).
CONCLUSIONS. Poor glycaemic control, dyslipidaemia and HT are common in diabetic children, putting them at risk of cardiovascular complications in adulthood.
Studies have reported that there has been an increase of 2 - 5% in the annual incidence of type 1 diabetes (T1D) worldwide.[1] In the Western Cape Province of South Africa (SA), the incidence of T1D is unknown, but is likely to be 5 new cases per 100 000.[2] The worldwide incidence of type 2 diabetes (T2D) in children and adolescents ranges from 1 to 51 per 1 000.[3] There is no estimate for SA, but its prevalence is likely to be on the increase.
The Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications Cohort[4] (DCCT/ EDIC) has shown that poor glycaemic control is associated with a potentially more atherogenic profile in adults. In this trial, small very low-density lipoprotein (VLDL), small high-density lipoprotein (HDL), medium low-density lipoprotein (LDL) (women), small LDL (men) and LDL particle size were directly correlated, while HDL particle size was negatively correlated, to HbA1c levels. A 28.6% incidence of dyslipidaemia and an 8.1 % incidence of hypertension were reported in a study of 27 358 children, adolescents and young adults with T1D.[5] In the UK, mortality rates have been shown to be much higher and increase earlier in T1D dyslipidaemic patients compared with non-diabetic dyslipidaemic patients.[6] The atherosclerotic process starts in childhood as evidenced by intima-media thickness of the carotids and aorta.[7-91 Aortic fatty streaks and fibrous plaques in the abdominal aorta and coronaries of young adults who died of suicide or homicide were associated with non-HDL cholesterol (nonHDL-C), hypertension (HT), impaired glucose tolerance and obesity, and inversely associated with HDL-C.[10] Mild to moderate hypertriglyceridaemia is known to be an independent risk factor for cardiovascular disease in adults[11] To the best of the authors' knowledge, this link has not been established for the paediatric age group. The 2018 guideline of the International Society of Paediatric and Adolescent Diabetes (ISPAD) recommends that a lipid profile should be done every 2 years and blood pressure (BP) measured annually in all children older than 11 years who have had diabetes for 2 - 5 years.[12] Screening should commence at 2 years of age if there is a family history of hypercholesterolaemia, early cardiovascular disease or if the family history is unknown.
In the Paediatric Diabetes Unit at Tygerberg Hospital (TBH) the practice has been to monitor fasting lipid profiles annually in all pre-pubertal diabetic children once they have been diagnosed for 5 years, and in all pubertal diabetic children once they have been diagnosed for 2 years. In a recently published paper[14] from the same unit, the introduction of a diabetes care team showed a decrease in HbA1c variability, hospital admission and diabetic ketoacidosis (DKA) rates. However, at 9.3%, the average HbA1c of all children was still unacceptably high. Presumably, this is associated with atherogenic lipid profiles and HT. A retrospective analysis of the lipid and BP surveillance programme was therefore performed, firstly, to determine the prevalence of dyslipidaemia and HT in paediatric diabetes patients seen at TBH; secondly, to establish whether dyslipidaemia and HT were associated with BMI, HbA1c and duration of disease; and thirdly, to determine whether lipid abnormalities and HT did improve over time, by comparing the first 5-year period (2007 - 2011) with the second (2012 - 2017).
Methods
All children and adolescents with diabetes, aged 1 - 19 years, seen at TBH between 2007 and 2017, were considered, but were only included if the diabetes duration was >5 years in pre-pubertal or >2 years in pubertal children. Pubertal assessment was done clinically. The clinical records were reviewed, and the following data collected: age; sex; weight; height; BP (measured with an automated BP monitor); type of diabetes; HbA1c; fasting serum total triglycerides; total cholesterol; HDL-C; and LDL-C. Only the most out-of-range lipid profile (with associated data) was recorded. Body mass index (BMI) percentiles (Centers for Disease Control and Prevention (CDC) criteria) were computed. BP readings were converted to percentiles.[14]
Definitions
• Dyslipidaemia: serum LDL-C >2.6 mmol/L or HDL-C <1.1 mmol/L or triglycerides (TG) >1.7mmol/L.[15]
• Hypertension: systolic and/or diastolic BP >95th percentile for sex, age and height percentile.[14]
• Poor glycaemic control: HbA1c >7.5%.[15]
• Overweight: BMI >85th percentile on the CDC chart.[16]
• Obesity: BMI >95th percentile on the CDC chart. [16]
• Adolescence: age >10 years.[17]
Statistical analysis
Descriptive statistics were calculated: means and standard deviations for continuous variables; and frequencies and percentages for categorical variables. Confidence intervals (CIs) (95%) were calculated and reported where relevant. The univariate associations between dyslipidaemia and HT and various demographic (age, gender, period) and clinical characteristics (type of diabetes, duration of disease, HT, BMI percentile and HbA1c) were evaluated using chi-square tests. A multiple logistic regression model was used to evaluate the effect of various risk factors on the probability of dyslipidaemia adjusted for age and sex. This model was also used to compare the 5-year periods (first 5 v. second 5 years) and to ensure that the confounders of age, sex, BMI percentile, type of diabetes and duration of diabetes were accounted for.
Ethical approval
Ethical approval was obtained from the Health Research Ethics Committee at Stellenbosch University (HREC ref. no. S17/10/229).
Results
Dyslipidaemia
A total of 145 T1D and 9 T2D patients were included in the study -all were adolescent and 8 of the 9 T2D patients were girls (Table 1). Of these, 57.8% (n=89/154; 95% CI 51.7 - 65.0) were dyslipidaemic. The prevalence of each respective lipid abnormality is shown in Fig. 1. A total of 66.7% (n=6/9; 95% CI 33.1 - 90.0) of the T2D patients presented with dyslipidaemia, and the respective figure for T1D was 57.2% (n=83/145; 95% CI 49.0 - 65.1). The prevalence was not significantly different between the diabetes types (p=0.58).
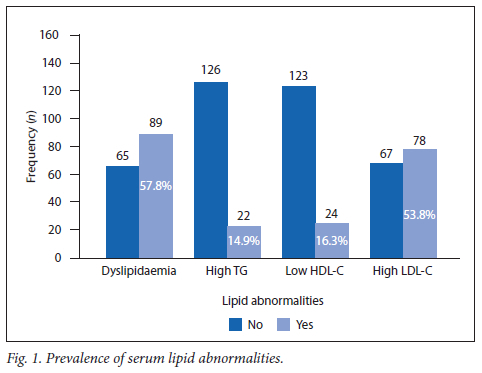
The majority (93.5%; n=144/154) of the children and adolescents with diabetes had poor glycaemic control. No statistical difference in lipid abnormality was seen between controlled or poorly controlled glycaemic patients (p=0.07).
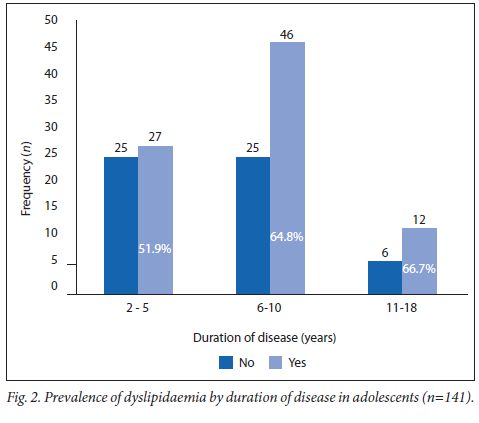
The prevalence of dyslipidaemia was 66.7% (n=12/18) in the adolescents with more than 10 years of diabetic disease (Fig. 2) compared with 64.8% (n=46/71) in the group with 6 - 10 years of disease and 51.9% (n=27/52) in the group with up to 5 years of disease (p=0.297). Of the poorly controlled adolescents, 62.6% (n=82/131) had associated dyslipidaemia (p=0.04) and, of these, 40.0% (n=60/154) were >16 years of age and 71.7% of them (n=43/60; 95% CI 60.9 - 81.7) were dyslipidaemic compared with 17 (28.3%; 95% CI 18.3 - 41.1) who were not (p=0.005).
There was no association between dyslipidaemia and BMI percentile (p=0.43). Seventeen patients (11.0%) were obese, of which 11 (64.7%) had an abnormal lipid profile (p=0.55).
In the second 5-year period, more than half of the children (54.8%) were dyslipidaemic (n=51/93; 95% CI 44.6 - 64.7) compared with 62.3% (n=38/61; 95% CI 49.5 - 73.5) in the first 5-year period (p=0.85). Different children (comparable in terms of HbA1c and BMI) were enrolled in each 5-year period.
Hypertension (HT)
Nearly half (48.7%; n=75/154; 95% CI 41.6 - 55.1) of the children and adolescents with diabetes were hypertensive. Of the overweight/ obese diabetics, 63.6% (n=35/55; 95% CI 50.2 - 75.3) were hypertensive compared with 36.4% (n=20/55; 95% CI 24.7 - 49.9) of those who were not overweight. Only 40.4% of diabetics of normal weight had HT (n=40/99; 95% CI 31.2 - 50.4), compared with 60.0% (n=59/99; 95% CI 49.6 - 68.9) who had no HT (p=0.01 for all four comparisons). More dyslipidaemic patients had HT than patients without dyslipidaemia and HT (61.3% (n=46/75; 95% CI 49.8 - 71.7) v. 45.6% (n=29/75; 95% CI 34.9 - 56.7), but the difference was not significant (p=0.39). The prevalence of HT between the two 5-year periods was 44.3% and 51.6%, respectively (p=0.37).
Discussion
Dyslipidaemia was seen in more than half of the children and adolescents with diabetes attending the paediatric diabetes clinic at TBH. Comparison of the present study with others is difficult owing to considerable heterogeneity in age groups, diabetes duration, BMI references and cut-offs, as well as lipid profiles. In the UK, 38% of children and adolescents with diabetes had high non-fasting LDL-C levels (>2.6 mmol/L) taken after a variable time interval.[18] In a German surveillance study, however, fasting-elevated LDL-C (>3.4 mmol/L) was seen in 11%,[5] while in a cross-sectional study in the USA 48% of diabetic youth reported a high fasting LDL-C (>2.6 mmol/L).[19] The last figure agrees with the present study, in which 53.8% of participants had fasting LDL-C levels of >2.6 mmol/L. Low HDL-C was seen in about 5% (HDL-C <0.9 mmol/L) of German, 23.7% (HDL-C <1.1 mmol/L) of UK and 44% (HDL-C <1 mmol/L) of US children and adolescents with diabetes compared with a prevalence of 16.3% of low HDL-C (HDL-C <1.2 mmol/L) in the current study. Thirty-nine percent of the US patients had an elevated serum TG level compared with 14.9% in the current study. The UK and German studies did not report TG values. The proportion of poorly controlled diabetics in the current study is considerably higher compared with the German study (93.5% v. 70%, respectively).[5] This could explain the higher prevalence of dyslipidaemia in the present study, since a relationship between total cholesterol and HbA1c has previously been demonstrated.[15]
The prevalence of dyslipidaemia was higher during adolescence, particularly beyond 16 years of age, which corresponds with Schwab et al.[5] Surprisingly, BMI percentile, and hence overweight/ obesity, was not associated with dyslipidaemia. Obesity is, however, one of the known risk factors for atherosclerosis in paediatric diabetes.[10,20,21] Overweight/obesity has also been shown to increase in diabetic adolescents with advancing age.[5] The small sample size of the current study is the most likely explanation for the lack of association between adiposity and dyslipidaemia. The lipid profiles of the patients in the second 5-year period tended to be less abnormal compared with the first, but the prevalence of dyslipidaemia was still unacceptably high. This, presumably, attests to poor glycaemic control and inadequate nutritional intervention which had not improved over the years. Duration of diabetes, increasing age and the universally poor glycaemic control (bar 6.5% of patients) compromise cardiovascular health of the adolescent diabetic population at TBH.
Just under half of the children and adolescents with diabetes in the study had HT, mirroring the prevalence of dyslipidaemia in this patient population. Renal function was not recorded during this study, but renal failure is unlikely to contribute to the high prevalence, because very few participants had documented microalbuminuria. In the German surveillance study, the prevalence of HT was a mere 10%.[5] The lower prevalence was most likely related to a lower prevalence of dyslipidaemia and obesity (7% v. 11% in the present study), and indirectly related to glycaemic control. Technical factors (e.g. using an automated BP monitor without manual confirmation) and the documentation of a single reading (rather than three) may have resulted in inflated cases of HT. As expected, more overweight/obese children were hypertensive, and there was a trend of more diabetics with an abnormal lipid profile having HT. Considerable efforts must be made to both prevent (by better glycaemic control) and treat HT. Notably, only a small number of patients were on medication for HT or dyslipidaemia.
Study limitations
The major limitation of this study was the small sample size. A multi-centre study may have provided results that could reach more definitive conclusions. Secondly, the pre-adolescent age group was particularly small, allowing for limited conclusions only. Thirdly, the local lipid screening practice may have led to some cases being missed, resulting in underreporting. Fourthly, nonHDL-C, lipoprotein particle size, and lipoprotein (a)[22] were not routinely measured, making the study less informative. Lastly, as with any retrospective study, missing data, inadequate documentation, and single BP measurements may have compromised the quality of the data.
Conclusions and recommendations
The prevalence of dyslipidaemia and HT is unacceptably high in children and adolescents with diabetes at TBH. Dyslipidaemia is most prevalent during adolescence, especially in adolescents above 16 years of age. BMI percentile was not associated with dyslipidaemia, but HT was more prevalent in overweight/obese patients. Glycaemic control was poor in nearly all patients. Dyslipidaemia, poor glycaemic control, and HT put these paediatric diabetic patients at risk of cardiovascular complications in adulthood. Efforts need to be intensified to manage these risk factors. Although a diabetes management team was introduced during the period of study, both the diabetes nurse educator and the dietician have many other commitments, resulting in suboptimal care for children with diabetes. Funds should be made available to employ both in the respective roles on a full-time basis. Furthermore, a multi-centre study should be performed to confirm whether the prevalence of dyslipidaemia and HT in adolescent diabetics reported here is seen countrywide.
Declaration. The manuscript was submitted in partial fulfilment of the requirements for an MMed (Paeds) degree (LND).
Acknowledgements. None.
Author contributions. LND: conducted study; wrote thesis. CJL: data analysis; editing draft. EWZ: Conceptualised study; data analysis; wrote paper.
Funding. None.
Conflicts of interest. None.
References
1. Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010;39(3):481-497 https://doi.org/10.1016/j.ecl.2010.05.011 [ Links ]
2. Robertson A. Chronic conditions in children. S Afr Health Rev 2006;15(1):257-270. [ Links ]
3. Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Curr Diab Rep 2014;14(8):508. https://doi.org/10.1007%2Fs11892-014-0508-y [ Links ]
4. Jenkins AJ, Lyons TJ, Zheng D, et al. Serum Lipoproteins in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications Cohort. Diab Care 2003;26(3):810-818. https://doi.org/10.2337/diacare.26.3.810 [ Links ]
5. Schwab KO, Doerfer J, Hecker W, et al. Spectrum and prevalence of atherogenic risk factors in 27 358 children, adolescents, and young adults with type 1 diabetes. Diab Care 2006;29(2):218-225. https://doi.org/10.2337/diacare.29.02.06.dc05-0724 [ Links ]
6. Laing SP, Swerdlow AJ, Carpenter M, et al. Mortality from cerebrovascular disease in a cohort of 23 000 patients with insulin-treated diabetes. Stroke 2003;34(2):418-421. https://doi.org/10.1161/01.STR.0000053843.03997.35 [ Links ]
7. Harrington J, Peña AS, Gent R, et al. Aortic intima media thickness is an early marker of atherosclerosis in children with type 1 diabetes mellitus. J Pediatr 2010;156(2):237-241. https://doi.org/10.1016/j.jpeds.2009.08.036 [ Links ]
8. Järvisalo MJ, Putto-Laurila A, Jartti L, et al. Carotid artery intima-media thickness in children with type 1 diabetes. Diabetes 2002;51(2):493-498. https://doi.org/10.2337/diabetes.51.2.493 [ Links ]
9. Järvisalo MJ. Endothelial dysfunction and increased arterial intima-media thickness in children with type 1 diabetes. Circulation 2004;109(14):1750-1755. [ Links ]
10. McGill HC Jr, McMahan CA, Henerick EE, et al. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr 2000;72(Suppl):1307S-1315S. https://doi.org.ez.sun.ac.za/10.1093/ajcn/72.5.1307s [ Links ]
11. Blom DJ. Elevated triglycerides: A matter of the heart and pancreas. S Afr Med J 2018;108(4):258-261. https://doi.org/10.7196/SAMJ.2018.v108i4.13235 [ Links ]
12. Donaghue KC, Marovecchio ML, Wadwa RP, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes 2018;19(Suppl 27):262-274. https://doi.org/10.1111./pedi.12742 [ Links ]
13. Kajee Z, Harvey J, Zöllner EW. The impact of a diabetes care team on the glycaemic control of paediatric and adolescent patients with type 1 diabetes mellitus at Tygerberg Children's Hospital. S Afr J Child Health 2019;13(1):11-16. https://doi.org/10.7196/SAJCH.2019.v13i1.1492 [ Links ]
14. Lurbe E, Agabiti-Rosei E, Cruikshank JK, et al. European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertension 2016;34(10):1887-1920. https://doi.org/10.1097/HJH.0000000000001039 [ Links ]
15. Donaghue KC, Wadwa RP, Dimeglio LA, et al. ISPAD Clinical Practice Consensus Guidelines 2014 Compedium. Microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes 2014;15(Suppl 20):257-269. https://doi.org/10.1111./pedi.12180 [ Links ]
16. US Preventive Services Task Force. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. Pediatrics 2010;125(2):361-367. https://doi.org/10.1542/peds.2009-2037 [ Links ]
17. World Health Organization. Adolescence: A period needing special attention. Recognizing adolescence. https://apps.who.int/adolescent/second-decade/section2/page1/recognizing-adolescence.html (accessed 10 June 2021). [ Links ]
18. Edge JA, James T, Shine B. Longitudinal screening of serum lipids in children and adolescents with type 1 diabetes in a UK clinic population. Diabet Med 2008;25(8):942-948. https://doi.org/10.1111/j.1464-5491.2008.02518.x [ Links ]
19. Kershnar AK, Daniels SR, Imperatore G, et al. Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: The Search for Diabetes in Youth study. J Pediatr 2006;149:314-319. https://doi.org/10.1016/j.jpeds.2006.04.065 [ Links ]
20. Weihrauch-Blüher S, Wiegand S. Risk factors and implications of childhood obesity. Curr Obes Rep 2018;7:254-259. https://doi.org/10.1007/s13679-018-0320-0 [ Links ]
21. Boyer BP, Nelson JA, Holub SC. Childhood body mass index trajectories predicting cardiovascular risk in adolescence. J Adolesc Health 2015;56(6):599-605. https://doi.org/10.1016/j.jadohealth.2015.01.006 [ Links ]
22. Saeedi R, Frohlich J. Lipoprotein (a), an independent cardiovascular risk marker. Clin Diab Endocrinol 2016;2(7):1-6. https://doi.org/10.1186/s40842-016-0024-x [ Links ]
 Correspondence:
Correspondence:
E W Zöllner
zollner@sun.ac.za
Accepted 16 September 2021














