Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.16 n.3 Pretoria Sep. 2022
http://dx.doi.org/10.7196/SAJCH.2022.v16.i3.1876
RESEARCH ARTICLE
Determinants of diarrhoeal disease in children living in low-income households in a periurban community in Cape Town, South Africa
M K HendricksI; M SamboII; R LaubscherIII; S PendleburyIV; L BourneV
IMMed (Paed), MTropPaed (LSTM); Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, South Africa
IIBSc, MPhil (MCH); South African Medical Research Council, Cape Town, South Africa
IIIBCom;South African Medical Research Council, Cape Town, South Africa
IVPhD; Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, South Africa
VPhD; South African Medical Research Council, Cape Town, South Africa
ABSTRACT
BACKGROUND. Water, sanitation and hygiene are critically important in reducing morbidity and mortality from childhood diarrhoeal disease and malnutrition in low-income settings.
OBJECTIVES. To assess the association of diarrhoeal disease with factors relating to domestic hygiene, the environment, sociodemographic status and anthropometry in children <2 years of age.
METHODS. This was a case-control study conducted in a periurban community 35 km from the centre of Cape Town, South Africa. The study included 100 children with diarrhoeal disease and 100 age-matched controls without diarrhoea, who were recruited at primary healthcare clinics. Sociodemographic status, environmental factors and domestic hygiene were assessed using a structured questionnaire; anthropometry was assessed using the World Health Organization's child growth standards. Univariate and multivariate logistic regression analyses were performed to identify the factors associated with diarrhoea.
RESULTS. The results of the univariate logistic regression showed significant susceptibility to diarrhoea in study cases compared with controls when the caregiver was >25 years old (odds ratio (OR) 1.82; 95% confidence interval (CI) 1.02 - 3.23; p=0.042); when children were in day care or cared for by a family member or a relative than when cared for by their mother (OR 1.97; 95% CI 1.06 - 3.65; p=0.032); and when the mothers were employed rather than at home (OR 2.23; 95% CI 1.21 - 4.12; p=0.01). Multivariate logistic regression analysis was used to identify predictors of diarrhoea, which entailed relaxing the inclusion criteria for the univariate analysis variables (p<0.25). The predictors significantly associated with diarrhoea were household problems relating to rat infestation (OR 2.44; 95% CI 1.13 - 5.28; p=0.027); maternal employment (OR 2.47; 95% CI 1.28 - 4.76; p=0.007); and children in day care or cared for by a relative (OR 2.34; 95% CI 1.21 - 4.54; p=0.01). Significantly more of the mothers who were employed than those who were unemployed had children in day care or cared for by a relative.
CONCLUSION. Practices relating to employment, childcare and the domestic environment were significant predictors of diarrhoea. Effective policy implementation on water, sanitation and domestic hygiene could prevent diarrhoeal disease and reduce its impact on children's growth, especially during the annual diarrhoeal surge season in this and similar periurban communities.
The third sustainable development goal (SDG) aims to ensure healthy lives and promote wellbeing at all ages. This entails ending preventable neonatal and child deaths and achieving a neonatal and under-5 mortality rate of 12 and 25 per 1 000 live births, respectively, by 2030.[1] In South Africa (SA), progress has been made in reducing the under-5 mortality rate (U5MR) from 79 to 32 per 1 000 live births between 2004 and 2017.[2] While the reduction in HIV-related deaths has made a major contribution to decreasing the U5MR, there is a need to build on these gains through the prevention and appropriate management of the other causes of child mortality.
Nationally, diarrhoeal disease is the third main cause of death, accounting for 16% of under-5 deaths;[3] the first national burden of disease study found that it accounted for 8.8% of disability-adjusted life years (DALYS).[4] According to the South Africa Demographic Health Survey of 2016,[5] the prevalence of diarrhoea nationally is estimated at 10% in children under 5 years old, the peak age being 6 - 23 months, which is consistent with that in previous studies.[5,6 In Western Cape Province, there is a lower prevalence of diarrhoea (5%) in children under 5 years than nationally.
Poverty in association with the lack of access to safe drinking water, improved sanitation and good hygiene practices (WASH) contributes to the high prevalence of and mortality from childhood diarrhoea.[7] Children in such environments are exposed to faecal-oral transmission of pathogens through contaminated water, hands, flies, soil and food, as outlined in the F-diagram (Fig. 1).[8,9] Nationally, it is estimated that 30% of children do not have access to piped water in the home and 18% do not have access to improved sanitation.[2]
The United Nations Children's Fund (UNICEF) conceptual framework outlines unhealthy environments, with lack of access to WASH as an underlying cause of malnutrition in children through recurrent diarrhoea, parasite infections and environmental enteric dysfunction (EED).[10] The relationship between diarrhoea and malnutrition is bidirectional: diarrhoea leads to malnutrition from reduced dietary intake, intestinal malabsorption and increased metabolism; and malnutrition through reduced immunity and increased susceptibility to enteric infection leads to severe and prolonged diarrhoea.[11] EED is a subclinical condition experienced by children living in unsanitary environments with high exposure to faecal pathogens. Chronic ingestion of these pathogens leads to T-cell-mediated intestinal changes, including villous atrophy, crypt hyperplasia, inflammation and increased permeability. The last change facilitates microbial translocation, which triggers an immune response and metabolic changes. It is proposed that these changes, malabsorption and a reduced dietary intake contribute to stunting in childhood.[12]
In reducing the impact of diarrhoea and EED on children's linear growth, the implementation of WASH interventions is critical. The impact of interventions such as water chlorination, sanitation, handwashing with soap along with infant and young child feeding (IYCF) was recently assessed in the WASH benefits and SHINE trials in Bangladesh, Kenya and Zimbabwe. The WASH interventions were found to have no effect on linear growth and when provided with IYCF had no additional benefit compared with IYCF alone.[13] This finding was mainly attributed to high enteropathogenic exposure from environmental faecal contamination, despite the implementation of the abovementioned interventions. The trials also showed that there were reductions in diarrhoeal disease through handwashing and point-of-use water treatment when there was frequent contact between health promoters and participants, which is consistent with studies where these interventions have been effective at the household level.
In poor environments where there is a high exposure of children to faecal pathogens, the provision of safe water and improved sanitation alone cannot prevent diarrhoeal disease. In these settings, hygiene practices are critically important in preventing faecal contamination of the home environment, reducing diarrhoeal disease and EED, and its impact on children's growth. A reduction in diarrhoea has been noted in randomised controlled trials on handwashing with soap and fly control; in observational studies of the safe disposal of human faeces, animal faeces and waste; and in studies relating to safe food preparation and storage.[14]
The current study aimed to determine the multiple risk factors associated with diarrhoea in children living in a low-income community with limited access to safe water and improved sanitation and where domestic hygiene practices were not known. It was envisaged that the identification of risk factors could inform interventions to prevent diarrhoea, especially during the annual diarrhoeal surge season. The objectives of the study were to assess the association of diarrhoea with domestic hygiene, environmental factors, sociodemographic status, feeding and anthropometry in children <2 years of age.
Methods
Study site
The study was performed over 6 months in the Khayelitsha subdistrict - Cape Town's largest township, situated ~35 km from the central business district (CBD). In 2013, the population, which is predominantly black African (99%), was estimated to be 391 748. Of the almost 120 000 households, 45% lived in formal dwellings, i.e. free-standing houses, flats, town or cluster houses constructed from bricks;[15] 55% lived in informal dwellings, i.e. shacks, some within backyards, and built from iron, wood and other non-durable materials; 62% had access to piped water, either in the dwelling or yard; 72% had access to flush toilets; 81% had their refuse removed weekly; and 81% used electricity.[16]
Study design, sampling and participants
This was a case-control study of children <2 years old with diarrhoea (cases) and without diarrhoea (controls). Cases and controls were selected at four primary healthcare clinics and a community healthcare centre located in different areas of the subdistrict. Using Epi Info statistical software (CDC, USA), a sample size of 200 (100 cases and 100 controls) was calculated to detect an odds ratio (OR) of 2.4, 80% power, 95% confidence interval (CI), assuming exposure of 30% in the controls and 50% in the cases.[17] An average of 3 cases and 3 controls was purposively selected each day during the study period. A case was defined as a child <2 years of age, living in the subdistrict and who attended the health facility with acute diarrhoea, with or without dehydration; it included the passing of >3 loose or watery stools per day.[18] Controls were healthy children attending the health facility for immunisations or minor injuries. Children who were HIV positive based on the HIV DNA polymerase chain reaction (PCR) test at 6 weeks of age, and those who were HIV exposed, were excluded from the study. Controls who had diarrhoea within 1 month before visiting the health facility were excluded.
Variables assessed and data collection
The exposure variables assessed in cases and controls included: (i) sociodemographic variables - child's age, gender; caregiver's age, marital status, educational level, employment status; household income; household size; type of house and number of rooms; household energy source; (ii) environmental variables - location of taps and water storage; type of toilet; waste storage and removal; (iii) domestic hygiene variables -household washing facilities; surface cleaning (e.g. tables and floors); handwashing with soap and water; presence of household insects and rodents; food preparation and child feeding; (iv) child feeding and anthropometric variables - child feeding, including exclusive breastfeeding, formula feeding and complementary feeding. Exclusive breastfeeding included breastmilk only with no liquids or solids, except for vitamins, minerals and medication; supplementary feeding included formula or breastmilk substitutes in addition to breastmilk; complementary feeding included solid or semi-solid food in addition to breastmilk. Anthropometric measurements included weight-for-age, length-for-age and weight-for-length. A trained field worker administered a structured questionnaire in both English and isiXhosa to caregivers. A pilot study was done prior to the main study and modifications were made to the questionnaire to ensure clarity and consistency of questions. All questionnaires were checked for corrections and completion after the interview by the researcher, who was a master's student.
In assessing the children's anthropometry, a Seca 354 electronic baby scale (Seca, USA) was used to measure weight to the nearest 0.1 kg. The accuracy of the weighing scales was checked daily against known weights. The child was weighed twice to ensure reliability. The length of the child was measured using a Seca 210 mobile measuring mat to the nearest 0.1 cm. The Seca 212 measuring tape was used to measure the head circumference of the child to the nearest 0.1 cm. All the anthropometric measurements were done by the researcher.
Data analysis
Data from completed questionnaires were entered into Excel (Microsoft Corp., USA). These data were checked for accuracy and consistency. Statistical analyses were performed using Stata 16 (StataCorp., USA).'191 Frequencies and cross tabulations were used to check for missing values. Univariate logistic regression analysis was done by comparing the outcome variable (diarrhoea) with each independent variable relating to sociodemographic status, environmental factors, domestic hygiene and children's feeding and anthropometry. The results were reported as ORs, 95% CIs and p-values. Variables with p<0.25 by univariate analyses were selected as predictors for inclusion in a stepwise multivariate logistic regression model. The set of possible predictors was then determined from the final logistic regression model to obtain the ORs, 95% CIs and p-values. A p-value <0.05 was regarded as statistically significant.
Analysis of children's anthropometry
The anthropometric results were obtained using WHO Anthro v3.2.2 of the World Health Organization's child growth standards. These results were copied to Stata 16 for further analysis. The z-scores for weight-for-age (WAZ), length-for-age (LAZ) and weight-for-height (WHZ) were categorised using the cut-off points <-2 standard deviation (SD) and >-2 SD; for overweight a cut-off of WHZ >+2 SD was selected.
Ethical approval
Ethical approval for the study was obtained from the Human Research Ethics Committee, Faculty of Health Sciences, University of Cape Town (ref. no. 104/2012) and the Department of Health, City of Cape Town (ref. no. 10294). The researcher and field worker explained the purpose of the research to each participant and a consent form was issued for each of them to sign. Confidentiality was maintained during the interview.
Results
The total study sample comprised 200 caregiver-infant pairs (100 cases and 100 controls). All 200 caregiver-infant pairs were included as participants, using the structured interview questionnaire.
Association between sociodemographic status and diarrhoea
The proportion of children in the case and control groups was similar in terms of age and gender (Table 1). The proportion of caregivers in the two groups was similar with regard to gender, education, marital status and income. The results of the univariate logistic regression showed significant susceptibility to diarrhoea in cases compared with controls when the caregiver was >25 years old (OR 1.82; 95% CI 1.02 - 3.23; p=0.042); when children were in day care or cared for by a family member or relative and not by their mothers (OR 1.97; 95% CI 1.06 - 3.65; p=0.032); and when the mothers were employed rather than at home (OR 2.23; 95% CI 1.21 - 4.12; p=0.01).
Association between diarrhoea and children's environment
Of the cases and controls, 41% and 30%, respectively, had taps inside the house, while the remainder had access to outside taps and stored water in containers (Table 2); 93% and 85% had flush toilets, while 7% and 15% used a bucket or septic toilet, respectively. Of cases and controls with flush toilets, 36% and 26%, respectively, were located inside the house. Of the cases and controls, 56% and 55% used closed bins, while 44% and 45% used municipal or plastic bags to store waste, respectively. In 56% and 55% of the case and control caregivers, respectively, there was weekly municipal waste removal, while the remainder carried plastic bags to the municipal community waste storage weekly. Caregivers in the case and control groups reported problems in their homes with rats (80% v. 73%), flies (56% v. 51%), fleas (55% v. 58%) and cockroaches (22% v. 27%). The univariate logistic regression analyses showed no significant associations between diarrhoea and any of the environmental variables in the two groups.
Association between domestic hygiene and diarrhoea
Of the caregivers in the case and control groups, >70% reported handwashing >6 times in the previous 24 hours (Table 2). Handwashing by caregivers in both groups was >90% after using the toilet and changing the baby's nappy; >80% before feeding the baby; >60% before eating; and almost 40% or more before cooking. Of the case and control groups, 36% and 27%, respectively, used a kitchen sink inside the house to wash utensils, while 64% and 73%, respectively, washed kitchen utensils in a container. The results of the univariate logistic regression analysis did not show a significant relationship between diarrhoea and the self-reported domestic hygiene behaviour variables.
Association between diarrhoea and child feeding and anthropometry
Of the case and control groups, 12% in each of the groups were exclusively breastfed; 16% and 19% were exclusively formula fed; 52% and 44% were mixed fed; and 20% and 25% were given complementary feeds, respectively. Of the caregivers in the case and control groups, 95% and 89%, respectively, fed their babies immediately after preparation of feeds and 98% and 92%, respectively, stored formula milk not consumed in a refrigerator. The univariate logistic regression results did not show significant relationships between diarrhoea and type of feeding, formula milk preparation and refrigeration of left-over formula milk.
Of the cases and controls, 1% and 5% were underweight; 12% and 27% were stunted; 4% and 0% were wasted; and 28% and 32% were overweight, respectively (Table 2). Stunting was significantly higher in controls than cases (χ2=6.2; p=0.012). The univariate logistic regression analysis showed that a LAZ <-2 SD was associated with a significantly lower risk of diarrhoea (OR 0.37; 95% CI 0.17 - 0.78; p=0.009).
Variables with p<0.25, and included as predictors in a multivariate logistic regression model, included caregiver's age, mother's employment, childcare during the day, location of taps inside or outside the house, type of toilet, pests in the house (rats), number of people living in the house, number of children <5 years old, washing of dishes in a kitchen sink or container, and using the same cloth for washing dishes and for cleaning surfaces.
Logistic regression model of factors associated with diarrhoea
The final multivariate regression model revealed a significant association with diarrhoea when the mother was employed (OR 2.47; 95% CI 1.28 - 4.76; p=0.007) (Table 3). Significantly more of the respondents who were employed than those who were unemployed had children in day care or cared for by a relative (n=48/63 (76.2%) v. n=12/137 (8.8%); p=0.00). As these two variables were confounders, they could not be used simultaneously in the same model. When assessed independently in the model, there was a significant association with diarrhoea when children were in day care or if cared for by a family member (OR 2.34; 95% CI 1.21 - 4.54; p=0.01). There was a significant association with diarrhoea with rats in the house (OR 2.44; 95% CI 1.13 - 5.28; p=0.023) (Table 3). There was a significantly lower association with diarrhoea in households with >6 people (OR 0.45; 95% CI 0.22 - 0.91; p=0.027); in households using a toilet outside the house (OR 0.39; 95% CI 0.19 - 0.83; p=0.015); and in a LAZ <-2 SD (OR 0.36; 95% CI 0.16 - 0.78; p=0.01).
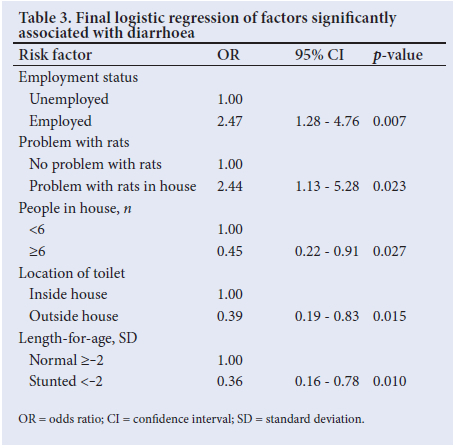
Discussion
WASH is critically important in reducing morbidity and mortality from diarrhoeal disease in low-income countries.[20] Most of the cases and controls in the current study had access to piped water, either inside or outside the house, and close to 90% had flush toilets. Access to an on-plot water supply probably facilitated handwashing, which was practised by >90% in both groups after using the toilet and changing nappies and by >80% before feeding. However, this study showed that there were significant predictors of diarrhoea relating to sociodemographic factors and domestic hygiene practices among the cases compared with controls.
Children had a significantly higher risk of diarrhoea when the mother was employed than when she was at home and caring for the child. This finding is consistent with that in a study on risk factors for diarrhoea in children from slum and non-slum areas in Dhaka, Bangladesh.[21] Children living in slum areas characterised by informal housing, limited access to safe water, sharing of toilets and whose mothers were employed had a significantly greater risk of diarrhoea than those from non-slum areas. The reason for this was that income-earning mothers from slum areas had less time for childcare, which together with the unsanitary environmental conditions contributed to a higher rate of diarrhoeal disease in their children.
In our study, there was a significantly higher risk of diarrhoea in the children whose mothers were employed and who were in day care or cared for by a family member. This finding is supported by the results of a case-control study of diarrhoea among children <3 years old in the USA, who were attending day care centres, receiving childcare within a home, or cared for by their caregivers. Children attending day care centres or receiving home care by another individual had significantly more episodes of diarrhoea than when they were cared for by their own caregivers.[22] The higher rates of diarrhoea in children attending day care centres or receiving childcare in homes relate to the excretion of enteric pathogens by non-toilet trained children <2 years of age, who may be asymptomatic. Transmission of pathogens to other children, personnel, toys and fomites was facilitated by close contact, and in the absence of infection control measures, outbreaks of diarrhoea occurred in these centres.[23]
A high proportion of cases and controls (80% v. 73%) reported household problems with rats, which was associated with a significantly higher risk of diarrhoea. The extent of the problem is consistent with that in a recent study on diarrhoea in children under 5 years old living in informal and formal settlements in Cape Town.[24] A study undertaken in rural Kenya on animal-related exposures to diarrhoea showed an increased risk of moderate to severe diarrhoea in children exposed to rodent excreta (urine and faeces) outside the house.[25] Exposure of children to rat excreta can result in diarrhoeal disease from Salmonella typhimurium, Escherichia coli and Cryptosporidium parvum. The study identified diarrhoeal pathogens in the faeces of domestic animals and found that children exposed to poultry and sheep were at a higher risk of diarrhoea, and that handwashing was protective after animal exposure.
Using an outside toilet by the children in our study was found to be protective against diarrhoeal disease. This finding was similarly found in a recent study in Cape Town, where a higher prevalence of diarrhoea occurred in children from formal settlements with access to private toilets and piped water compared with informal settlements with access to shared outside toilets and taps.[24] Despite a high number of households sharing the same toilet facility, there was a significantly lower risk of diarrhoea in the study participants. The reason for the lower risk of diarrhoea with the use of shared toilet facilities is not clear and requires further investigation. This is contrary to the findings of another study that showed a decrease in the quality of sanitation facilities with an increase in household use.[26]
An interesting finding was that households with >6 members were protected against diarrhoea. A study in Uganda showed that in households with 10 - 15 people there were 7 times greater odds of diarrhoea than in households with fewer people.[27] In our study, most of the case and control households had <5 people, with only 4 cases and 3 controls having >10 household members, which could explain the relative protective effect against diarrhoea in larger households.
In terms of the cases and controls, no significant differences were noted in acute malnutrition or wasting; stunting was significantly higher in the controls, which probably contributed to it showing a protective effect in relation to diarrhoea. The reasons for the higher stunting levels in the controls are not clear and the lower risk of diarrhoea in stunted children needs to be interpreted with caution, as previous studies have shown an association between diarrhoea and malnutrition. In a pooled analysis of 9 longitudinal studies on diarrhoea and growth, it was shown that 25% of stunting at 24 months could be attributed to >5 diarrhoeal episodes within the first 2 years of life.[28] A study undertaken in Peru found that, while diarrhoea could explain 16% of stunting, the lack of access to improved water and sanitation could explain 40% of stunting. In terms of the latter finding, environmental enteric dysfunction was proposed as the mediating factor between poor WASH conditions and impaired linear growth.[20]
As outlined in the UNICEF conceptual framework, reductions in stunting require improvements in WASH interventions, underpinned by effective governance of resources and services.[10] A recent systematic review found no studies or evidence linking governance, WASH and children's nutritional status from sub-Saharan Africa.[29 Inadequate governance relating to WASH in terms of stakeholders, managerial capacity, financial resources, and administration could limit achieving the SDG 6. The authors recommend integrating evidence-based information on children's nutritional status, WASH and governance into relevant policies. The implementation of these policies at a local level could ensure the effective delivery of WASH programmes, including environmental control and the counselling and education of caregivers on domestic hygiene.
Study limitations
The selection of an appropriate comparison group, as in other case-control studies, was difficult. The controls were matched to cases by age and gender. There could have been the potential for selection bias and as this is less of a problem in population-based case-control studies, neighbourhood rather than clinic controls may have been a preferable choice.
Conclusion
Practices relating to employment, childcare and the domestic environment were significant predictors of diarrhoea. Effective policy implementation on water, sanitation and domestic hygiene could prevent diarrhoeal disease and reduce its impact on children's growth, especially during the annual diarrhoeal surge season in this and similar periurban communities.
Declaration. None.
Acknowledgements. Dr Ariane de Lannoy for co-ordinating the funding and her support for the study; Dr Thabi Maitin and Ms Lebo Montewa of the South African Medical Research Council for facilitating the research intern funding and their support; the caregivers and children for participating in the study; health workers and facility managers at primary healthcare facilities in Khayelitsha for their cooperation and assistance; and Ms Khosi Jelwana for assisting with the data collection.
Author contributions. MKH wrote the manuscript and supervised and contributed to the study design, writing of the protocol and supervision of the research; MS contributed to the study design, wrote the study protocol, undertook the field research and contributed to the writing of the manuscript; LB contributed to the study design, study protocol and writing of the manuscript; SP contributed to the writing of the manuscript; RL undertook the data analyses and contributed to the writing of the manuscript.
Funding. The study was funded by a grant from the South Africa-Netherlands Research Programme on Alternatives in Development (SANPAD).
Conflicts of interest. None.
References
1. United Nations General Assembly. Transforming our World: The 2030 Agenda for Sustainable Development. Outcome Document of the United Nations Summit for the Adoption of the Post-2015 Agenda. New York: UN, 2015. [ Links ]
2. Lake L, Shung-King M, Hendricks M, et al. Prioritising child and adolescent health: A human rights imperative. S Afr Child Gauge 2019:32-53. [ Links ]
3. Nannan N, Groenewald P, Pillay-van Wyk V, et al. Child mortality trends and causes of death in South Africa, 1997 - 2012, and the importance of a national burden of disease study. S Afr Med J 2019;109(7):480-485. https://doi.org/10.7196/samj.2019.v109i7.13717 [ Links ]
4. Bradshaw D, Groenewald P, Laubscher R, et al. Initial burden of disease estimates for South Africa, 2000. S Afr Med J 2003;93(9):682-688. [ Links ]
5. National Department of Health, Statistics South Africa, South African Medical Research Council, ICF. South Africa Demographic and Health Survey 2016. Pretoria: NDoH, 2019. [ Links ]
6. Awotiwon OF, Pillay-van Wyk V, Dhansay A, Day C, Bradshaw D. Diarrhoea in children under five years of age in South Africa (1997 - 2014). Trop Med Int Health 2016;21(9):1060-1070. https://doi.org/10.1111/tmi.12739 [ Links ]
7. Mulugeta T. Socioeconomic, Environmental and Behavioural Factors Associated with the Occurrence of Diarrhoeal Disease among Under-five Children, Meskanena Mareko Woreda, Southern Ethiopia. Addis Ababa: Addis Ababa University, 2003. [ Links ]
8. Bourne LT, Pilime N, Sambo M, Behr A. Food hygiene and sanitation in infants and young children: A paediatric food-based dietary guideline. S Afr J Clin Nutrition 2013;26:S156-S164. [ Links ]
9. Wagner EG, Lanoix JN. Excreta Disposal for Rural Areas and Small Communities. Geneva: World Health Organization, 1958. [ Links ]
10. World Health Organization. Improving Nutrition Outcomes with Better Water, Sanitation and Hygiene: Practical Solutions for Policies and Programmes. Geneva: WHO, 2015. [ Links ]
11. Brown KH. Diarrhea and malnutrition. J Nutrition 2003;133(1):328S-332S. https://doi.org/10.1093/jn/133.1.328S [ Links ]
12. Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 2009;374(9694):1032-1035. https://doi.org/10.1016/s0140-6736(09)60950-8 [ Links ]
13. Pickering AJ, Null C, Winch PJ, et al. The WASH benefits and SHINE trials: Interpretation of WASH intervention effects on linear growth and diarrhoea. Lancet Glob Health 2019;7(8):e1139-e1146. https://doi.org/10.1016/S2214-109X(19)30268-2 [ Links ]
14. Curtis V, Schmidt W, Luby S, Florez R, Toure O, Biran A. Hygiene: New hopes, new horizons. Lancet Infect Dis 2011;11(4):312-321. https://doi.org/10.1016/s1473-3099(10)70224-3 [ Links ]
15. Hall K. Children's access to housing. S Afr Child Gauge 2019:248-251. [ Links ]
16. City of Cape Town. 2011 Census Suburb Khayelitsha. Cape Town: City of Cape Town, 2013. [ Links ]
17. Maponga BA, Chirundu D, Gombe NT, Tshimanga M, Shambira G, Takundwa L. Risk factors for contracting watery diarrhoea in Kadoma City, Zimbabwe, 2011: A case control study. BMC Infect Dis 2013;13(1):567. https://doi.org/10.1186/1471-2334-13-567 [ Links ]
18. World Health Organization, UNICEF. Diarrhoea: Why Children are Still Dying and What can be Done. Geneva: WHO, 2009. [ Links ]
19. StataCorp. Stata Statistical Software: Release 16. College Station: StataCorp., 2019. [ Links ]
20. Brown J, Cairncross S, Ensink JHJ. Water, sanitation, hygiene and enteric infections in children. Arch Dis Childhood 2013;98(8):629. https://doi.org/10.1136/archdischild-2011-301528 [ Links ]
21. Ferdous DS, Ahmed S, Farzana FD, et al. Diarrhoea in slum children: Observation from a large diarrhoeal disease hospital in Dhaka, Bangladesh. Trop Med Int Health 2014;19(10):1170-1176. https://doi.org/10.1111/tmi.12357 [ Links ]
22. Reves MA, Bartlett AV, Caruso CJ, et al. Child day care increases the risk of clinic visits for acute diarrhea and diarrhea due to rotavirus. Am J Epidemiol 1993;137(1):97-107. https://doi.org/10.1093/oxfordjournals.aje.a116607 [ Links ]
23. Pickering LK, Bartlett AV, Woodward WE. Acute infectious diarrhea among children in day care: Epidemiology and control. Rev Infect Dis 1986;8(4):539-547. https://doi.org/10.1093/clinids/8.4.539 [ Links ]
24. Nguyen TYC, Fagbayigbo BO, Cisse G, et al. Diarrhoea among children aged under five years and risk factors in informal settlements: A cross-sectional study in Cape Town, South Africa. Int J Environ Res Public Health 2021;18(11):6043. https://doi.org/10.3390/ijerph18116043 [ Links ]
25. Conan A, O'Reilly CE, Ogola E, et al. Animal-related factors associated with moderate-to-severe diarrhea in children younger than five years in western Kenya: A matched case-control study. PLoS Negl Trop Dis 2017;11(8):e0005795. https://doi.org/10.1371/journal.pntd.0005795 [ Links ]
26. Simiyu S, Swilling M, Cairncross S, Rheingans R. Determinants of quality of shared sanitation facilities in informal settlements: Case study of Kisumu, Kenya. BMC Public Health 2017;17(1):68. https://doi.org/10.1186/s12889-016-4009-6 [ Links ]
27. Omona S, Malinga GM, Opoke R, Openy G, Opiro R. Prevalence of diarrhoea and associated risk factors among children under five years old in Pader District, northern Uganda. BMC Infect Dis 2020;20(1):37. https://doi.org/10.1186/s12879-020-4770-0 [ Links ]
28. Checkley W, Buckley G, Gilman RH, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol 2008;37(4):816-830. https://doi.org/10.1093/ije/dyn099 [ Links ]
29. Momberg DJ, Ngandu BC, Voth-Gaeddert LE, et al. Water, sanitation and hygiene (WASH) in sub-Saharan Africa and associations with undernutrition, and governance in children under five years of age: A systematic review. J Dev Orig Health Dis 2021;12(1):6-33. https://doi.org/10.1017/s2040174419000898 [ Links ]
 Correspondence:
Correspondence:
M K Hendricks
michael.hendricks@uct.ac.za
Accepted 18 August 2021














