Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.16 n.3 Pretoria Sep. 2022
http://dx.doi.org/10.7196/SAJCH.2022.v16i3.1842
RESEARCH ARTICLE
Surgical treatment of bronchiectasis in children: An 11-year experience at a central health facility in KwaZulu-Natal, South Africa
M HbishI; J ChenI; P M JeenaII
IMMed (Cardiothoracic Surgery), FC Cardio (SA); Department of Cardiothoracic Surgery, Inkosi Albert Luthuli Central Hospital, Durban, South Africa
IICert Pulmonology (SA) Paed, PhD; Department of Paediatrics and Child Health, Inkosi Albert Luthuli Central Hospital, Durban, South Africa
ABSTRACT
BACKGROUND. The surgical management of children with bronchiectasis has seldom been reported.
OBJECTIVE. To describe the presentation, surgical management and outcomes in children with bronchiectasis presenting for surgery.
METHODS. We retrospectively reviewed the electronic records of 0 - 13-year-old children who underwent pulmonary resection for bronchiectasis at Inkosi Albert Luthuli Central Hospital, Durban, South Africa, between January 2004 and December 2014. Clinical, radiological and preoperative bronchoscopic findings, as well as surgical and histological outcomes, were analysed.
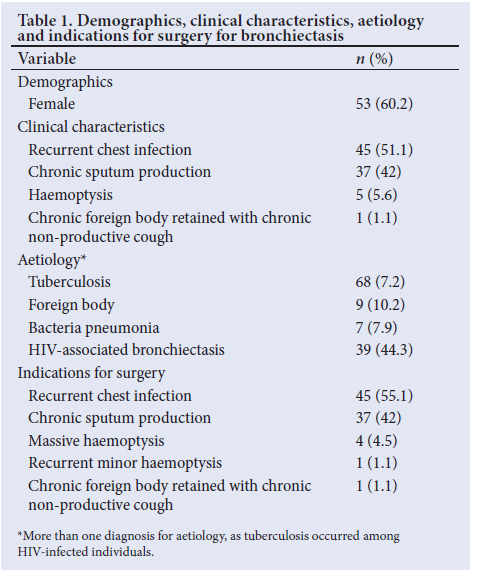
RESULTS. Eighty-eight patients underwent surgical resection. The female/male ratio was 3:2, with a mean age at surgery of 8.2 (range 2 - 13) years; 39 patients were HIV infected and 39 were HIV uninfected. Tuberculosis (TB) (n=68; 77.2%) was the most common cause of bronchiectasis, and recurrent chest infection (n= 45; 51.1%) was the most common clinical finding. Radiological examination confirmed isolated left-sided disease in 40 children (45.4%), isolated right-sided disease in 28 (31.8%) and bilateral disease in 20 (22.7%). Saccular disease with fibrocavitation (n=35; 39.7%) was the most common morphological disease type. Preoperative bronchoalveolar lavage samples confirmed a bacterial cause in 27 patients (30.6%). The most common operative procedures were primary pneumonectomy in 33 patients (37.0%), lobectomy in 30 (34.0%) and bilobectomy in 13 (14.7%). Seventy-five patients were asymptomatic after the operation and complications occurred in 13. Two children (2.2%), one with sepsis and the other with intraoperative hypoxia, died. Seventy patients underwent complete resection. At 1 month after surgery, 89.2% of patients were asymptomatic, while 77.7% of symptomatic patients were HIV positive.
CONCLUSIONS. Complete pulmonary resection in children with advanced-stage bronchiectasis is safe, with a low morbidity and mortality. Surgery in HIV-positive patients was not associated with worse outcomes and is not contraindicated. HIV- and TB-preventive measures could reduce the burden of childhood bronchiectasis.
At the beginning of the 19th century, Laennec first described permanent dilatation of the 2nd - 6th division of bronchi as bronchiectasis.[1] Since then, the incidence of bronchiectasis in children aged 0 - 14 years has varied widely from 0.5 to 1 500 per 100 000 child-years.[2] This variance was related to different populations studied, the astuteness of the physician in suspecting the diagnosis and the availability of diagnostic modalities such as highresolution computed tomography (HRCT) scans. With approved access to diagnostic modalities, early diagnosis, appropriate use of antimicrobial therapy and preventive strategies, there has been a decline in the overall incidence of the disease worldwide.[3] In developing countries, the incidence of bronchiectasis remains high and continues to be a major cause of morbidity (9.4 - 24.6%) and mortality (0 - 8.3%)>5] In South Africa (SA), the burden of disease is still unknown.[6,7]
The gold standard for defining bronchiectasis is the anatomical visualisation of the typical radiological changes of cystic dilatation of the bronchi. In patients without the characteristic cystic dilatation on chest radiographs, an HRCT scan is a reliable non-invasive method for assessing the degree of bronchial wall dilatation. The morphological characteristics of these changes can be classified into cylindrical, varicose or saccular, according to Reid's criteria.[8] In developed countries, cystic fibrosis (CF) is the most common cause of childhood bronchiectasis, while in developing countries, recurrent or persistent pneumonia secondary to Staphylococcus aureus, Bordetella pertussis measles or undetected foreign body aspiration is a common cause.[9-11] In KwaZulu-Natal, where tuberculosis (TB) and HIV infection are endemic, there is a greater risk for developing bronchiectasis secondary to these aetiological agents.
Early detection and appropriate medical treatment with antimicrobials, physiotherapy (postural drainage) and nutritional supplementation help with the prevention of disease progression. The administration of vaccines, chemoprophylaxis, avoidance of tobacco smoke, nutritional support (vitamins and trace elements), adequate adherence to antiretroviral and antituberculosis therapy and regular follow-up should prevent the development of advanced disease.[12] Acute exacerbations with community-acquired pathogens commonly occur in patients with bronchiectasis, and timely appropriate therapy for these infections reduces progression of disease. Prime indications for pulmonary resection in cases of bronchiectasis are the presence of chronic cough with excessive purulent sputum and haemoptysis. Both conditions are associated with an increased likelihood of spread to unaffected regions of the lung. Pneumonectomy, lobectomy or segmentectomy are all commonly performed with the aim of removal of diseased lung and improvement of quality of life.
Postoperative complication rates for bronchopleural fistula with associated empyema are seen in 9.1% of cases.[3] Reduced lung volumes with rib crowding have been reported on postoperative chest radiographs.
The surgical management of bronchiectasis in children from developing countries has not been adequately described and its role in management remains controversial.[13,14] We therefore undertook a retrospective review of all children with bronchiectasis who underwent pulmonary resection at a central referral hospital over an 11-year period to evaluate the clinical presentation, surgical methods and outcomes.
Methods
This retrospective descriptive study included all children (<13 years old) who underwent pulmonary resection for bronchiectasis at the Department of Cardiothoracic Surgery, Inkosi Albert Luthuli Central Hospital (IALCH), from 1 January 2004 to 31 December 2014. This is the only public health service unit for children and adults living in the provinces of KwaZulu-Natal and Eastern Cape. The department has 72 general beds, 8 high-care unit (HCU) beds and 8 intensive care unit (ICU) beds.
Records of all patients seen at IALCH are kept electronically and data were extracted from these records. Patients were entered into the study from the operative surgical register, taking age and diagnosis of bronchiectasis during the study period into account. Demographic data (age, sex, nutritional status, immune status (HIV/CD4 count/viral load) and clinical presentation were captured onto an Excel spreadsheet (Microsoft Corp., USA). The aetiology of bronchiectasis was determined by assessment of risk factors, microbiological findings on sputum and bronchoalveolar aspirate cultures, bronchoscopic findings before surgery, and intraoperative and histological findings. The diagnosis of pulmonary TB was based mainly on clinical evaluation and features on the chest radiograph and HRCT scan, and on microbiological and/or histological results, if available.
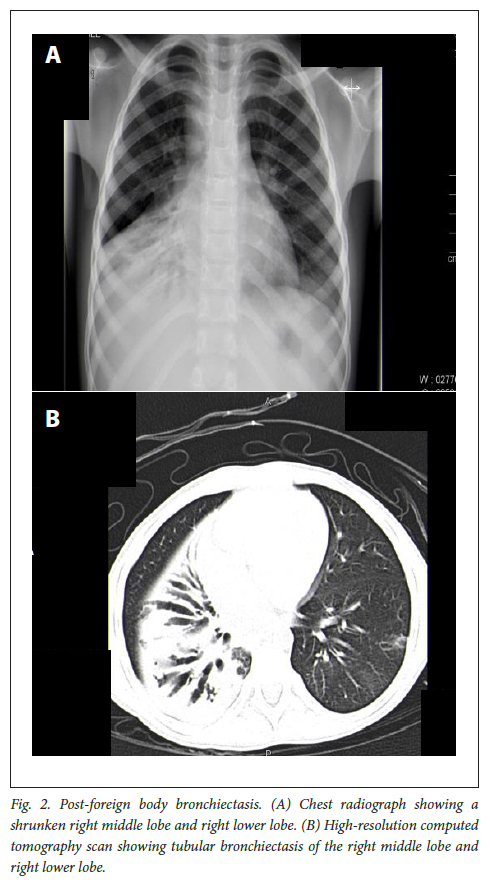
Posteroanterior and lateral chest radiographs and HRCT scans were evaluated to confirm the diagnosis, to determine the extent, localisation and morphology of the bronchiectasis and to exclude a foreign body. Figs 1 and 2 show the chest radiographs and HRCT scans of individual patients with post-TB and post-foreign body bronchiectasis, respectively. The diagnosis of bronchiectasis was confirmed in all patients on HRCT scans of the chest, with a bronchoarterial ratio >0.8 and lack of bronchial tapering.[2] From 2004 to 2009, pulmonary function results were not captured on the electronic record system and subsequent lung function values were not analysed owing to lack of records on technical accuracy. The haematological (full blood count (FBC)/erythrocyte sedimentation rate (ESR)) and biochemical laboratory results (albumin) were collated onto the Excel spreadsheet.

Standard medical treatment included a 6-month course of anti- TB therapy before surgery for all patients with a suspected diagnosis of TB, antiretroviral treatment where indicated, antibiotic therapy, physiotherapy, bronchodilators, nutritional support and repeated bronchoscopy for drainage. Indications for surgical treatment included localised disease on HRCT scan, chronic sputum production, recurrent chest infection and massive or recurrent minor haemoptysis. Intraoperative surgical care included a rigid bronchoscopy for lung toilet and locating the site of bleeding in all patients. Lung resections were undertaken via a posterolateral thoracotomy and whenever possible a muscle-sparing thoracotomy.
The chest cavity was entered via the fourth or fifth intercostal space. A bronchial blocker was used for lung isolation to prevent spillage of infected secretions to the contralateral lung and the anaesthesiologist practised frequent bronchial toilet.
Complete resection was defined as resection of all affected segments. Excessive bronchial dissection was avoided during surgery. The bronchial stump was closed manually by using interrupted Vicryl sutures. The stump was tested for air leaks and covered with mediastinal pleura. A single chest drain was inserted for cases of pneumonectomy and two chest drains for lobectomy or bilobectomy. In bilateral disease, the area with more extensive bronchiectasis, mycetoma or more pus at bronchoscopy was removed first. The second resection was undertaken after full recovery in 1 - 4 months after the first. Postoperative bronchoscopy with suctioning to dryness was performed.
All resected specimens were submitted for histopathological examination. Postoperative management included monitoring of all patients in paediatric HCUs for at least 48 hours, pain control, intensive chest physiotherapy, administration of antibiotics and early mobilisation. After discharge, patients were followed up every month for the first 3 months and thereafter every 6 months in our outpatient clinic, where their residual disease was assessed.
For study purposes, surgical indications, types of operation and postsurgical complications were recorded.
Statistical analysis
Data were entered on SPSS version 23 (IBM Corp., USA). Descriptive statistical analysis of the data (means, standard deviations, ranges, frequencies and percentages) was conducted prior to the χ2 test of association between categorical variables; p<0.05 was considered statistically significant.
Ethical approval
Ethical approval was granted by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (ref. no. BE032/17). Confidentiality was assured by password protection on all patient information files.
Results
Eighty-eight patients with operative bronchiectasis were identified over the 11-year study period (2004 - 2014). The mean age of the study participants was 8.2 (range 2 - 13) years. There were more female (n=53) than male patients in the study cohort, with a female:male ratio of 3:2. There were 39 HIV-positive children (44.3%), 39 HIV-negative children (44.3%) and in 10 cases the HIV status was not determined (11.3 %). Of the HIV-positive patients, 28 had their CD4 count assessed (71.8%), with a mean of 817 (range 134 - 2 034) cells/μL. Only 25 HIV-positive children received antiretrovirals; these were not administered to any of the HIV-positive children during the early part of the study, as all had undetected HIV viral loads. HIV viral load tests of the remaining untreated HIV-positive patients were not performed.
Clinical TB was the most common causative agent identified in the cohort (n=68; 77.2%), with 60 patients (68.1%) completing treatment before surgery and 8 (9.0%) receiving treatment during the current admission. HIV-associated recurrent opportunistic infections (n=39; 44.3%) were also commonly seen (Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis and Candida albicans). Foreign body inhalation and recurrent bacterial infection were seen in 9 (10.2%) and 7 (7.9%) patients, respectively (Table 1). All patients were symptomatic on presentation for surgery, with recurrent chest infection (n=45; 51.1%) and chronic sputum production (n=37; 42.0%) being the most frequent symptoms. Children rarely presented with haemoptysis (n=5; 5.6%) (Table 1).
The mean Hb for all enrolled patients was 11.5 (range 7.9 - 15.2) g/dL. The mean ESR level was 27.04 (range 8 - 152) mm/h, with 7 cases having levels >100 mm/h, 43 between 30 and 100 mm/h and 30 with levels <30 mm/h. The mean serum albumin was 41.7 (range 27 - 50) g/dL, with only 2 patients having levels <30 g/dL.
All patients underwent chest radiography. Preoperative chest radiography showed left-sided disease in 40 (45.4%) and right-sided disease in 28 (31.8%). The disease was unilateral in 68 (77.2%) and bilateral in 20 (22.7%) patients. Typical features of advanced bronchiectasis with atelectasis (n=53; 60.2%), fibrosis (n=44; 49.4%) and compensatory hyperinflation (n=37; 41.6%) were seen. Postoperative chest radiography confirmed reduced lung volumes and rib crowding, without any complications.
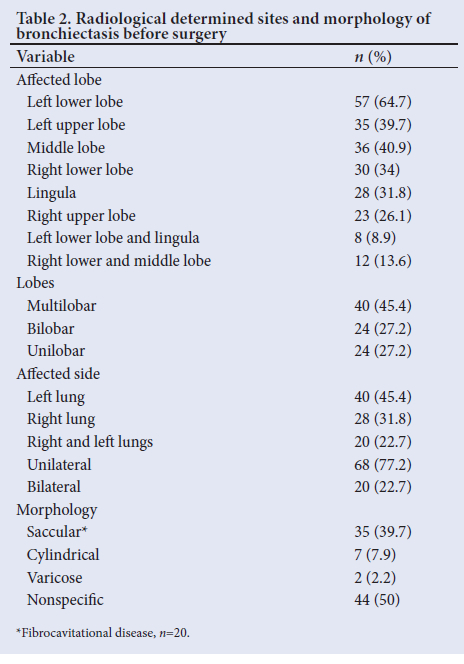
The diagnosis of bronchiectasis was confirmed in all patients after an HRCT scan of the chest. Presurgical HRCT scans confirmed multilobar disease in 40 patients (45.4%) - 16 in the left lung, 11 in the right lung and 13 in both lungs. Bilobar disease was seen in 24 patients (27.2%) - 9 in the left lung, 8 in the right lung and 7 in both lungs. Single-lobe disease was seen in 24 patients (27.2%), with the left lower lobe involved in 13 cases, 1 each in the left upper lobe and lingual segment, 4 in the right middle lobe, 3 in the right lower lobe and 2 in the right upper lobe. In total, the most common sites involved were the left lower lobe in 57 (64.7%), left upper lobe in 35 (39.7%) and right middle lobe in 36 (40.9%) (Table 2). The morphological appearance of the disease was described after HRCT scans in 44 patients (50.0%), with saccular disease seen in 35 (39.7%), of whom 20 had fibrocavitational disease, 7 (7.9%) cylindrical disease and 2 (2.2%) varicose disease (Table 2). Partial aerated segmental bronchiectasis and partial central aeration were seen in 8 and 2 patients, respectively, while pleural disease was seen in 8.
All patients underwent rigid bronchoscopy and bronchoalveolar lavage preoperatively. Bronchoscopy findings were: pus in 46 cases (left main bronchus (n=27; 30.6%), right main bronchus (n=24; 27.2%), right lobe (n=10; 11.4%), left lobe (n=4; 4.5%), right upper lobe (n=2; 2.2%) and left upper lobe (n=2; 2.2%)), mucoid secretions in 14 cases (15.9%), foreign bodies in 5 (5.7%), blood in 3 (3.4%) and clear secretions in 19 (21.6%). The bronchoalveolar lavage cultures were positive for bacteria in 27 patients (30.6%) and fungi in 5 patients.[56] H. influenzaewas seen in 16 patients (18.1%), S. pneumoniaein 8 (9.0%), C. albicansin 4 (4.5%) and Mycobacterium tuberculosis in 2 (2.2%), while single cases of M. catarrhalis and yeast (1.1%) were also observed (Table 3). All patients had good-quality bronchoalveolar lavage samples, with minimal epithelial cells and significant leucocytes.

The indication for pulmonary resection was recurrent chest infection in 45 patients (51.1%), chronic sputum production in 37 (42.0%), massive haemoptysis in 4 (4.5%), recurrent minor haemoptysis in 1 patient (1.1%) and chronic foreign body retained with chronic non-productive cough in 1 (1.1%) (Table 1).
Thoracotomy was performed in all patients - 33 (37.5%) underwent a pneumonectomy, 30 (34.0%) a lobectomy, 13 (14.7%) a bilobectomy, 8 (9.0%) a lobectomy and segmentectomy, 2 bilateral resection and 2 completion pneumonectomy (Table 4). Seventy patients (79.5%) underwent a complete resection and 18 (20.4%) incomplete resection. Of the 35 patients in whom pneumonectomies were performed, the left lung (n=22) was resected more commonly than the right lung (n=13). Lobectomy was performed in 51 patients, with a single lobe in 30 (19 predominantly in the left lower lobe) and 2 lobes in 21 patients (12 in the right middle lobe and right lower lobe, 8 in the left lower lobe and lingual segments, and 1 in the right upper lobe and right lower lobe).
In all 88 patients bronchiectasis was confirmed on histological examination; 84 had chronic non-active disease, 3 had active infection and 1 had TB. Histology confirmed fibrocavitational disease in 20 (22.7%), cystic or saccular disease in 15 (17.0%) and cylindrical tubular disease in 7 (7.9%).
Seventy-five patients were asymptomatic postoperatively, 13 had complications, 7 persistent pneumonia, 2 atelectasis and 1 each empyema and superficial wound infection (Table 4), while 2 children (2.2%), 1 with brain death secondary to intraoperative hypoxia (day 5) and 1 with sepsis (day 9), died. Seventy ofthe 75 asymptomatic patients had undergone complete resection, while 18 had residual disease. At 1 month postoperatively, 84 patients (95.4%) were seen; 9 (10.2%) with persistent residual disease (mainly HIV-infected children (n=7; 77.7%)). There was no difference between HIVpositive and HIV-negative children with regard to their presentation, aetiology and complications of surgery. At 6-month follow-up, 46 (52.2%) were healthy and the remainder were lost to follow-up.
Discussion
Surgical therapy for advanced bronchiectasis appears to be beneficial and safe in improving quality of life. In our study, 80% of children underwent complete primary surgical resection. At 1-month postoperative follow-up, 85% were asymptomatic despite extensive involvement of the lungs. Our complication rate of 14.8% and mortality rate of 2.2% were similar to those seen in the literature from developed countries, where surgery has been used to remove all bronchiectatic tissue and preserve lung parenchyma.[4,5] Surgery is useful to remove localised disease not controlled by medical treatment, treat haemoptysis and avoid cardiorespiratory restriction.[14,15] Surgery in HIV-infected patients was not associated with worse outcomes than in non-HIV-infected patients; therefore, surgery is not contraindicated.
In our experience, pneumonectomy appeared to be safe, even though there have been reports of it being a high-risk technical procedure with postoperative complications.[16,17] Surgery for bilateral bronchiectasis was rarely performed in our cohort, but also produced satisfactory results.[18,19] Indications for surgery in children with bronchiectasis have not been clearly established. In cases of TB and in HIV-endemic areas, persistent lung damage after treatment is often an indication for surgery.[13] Irregular adherence to therapy, lack of follow-up and poor socioeconomic status lead to progressive advanced disease. Sanderson et al.[20] showed better outcomes with surgical than medical treatment. There are, however, no randomised controlled trials assessing outcomes in patients who failed medical treatment and consequently had surgical resection than in those who continued with medical treatment.[21]
In our study, the most frequent cause of bronchiectasis was recurrent or persistent lung infection, similar to other studies from developing countries, but different to the developed world, where CF is the dominant cause.[9,10] As CF was considered to be uncommon in the African population during the study period, routine testing for CF was not instituted. Furthermore, once a specific diagnosis was suggested or confirmed, other investigations, particularly for primary immune deficiency, were not performed. TB, HIV-associated opportunistic infections, lymphoid interstitial pneumonitis, postinfectious causes (H. influenzae, S. pneumoniae, S. aureus, B. pertussis measles) and foreign bodies are the common causes of bronchiectasis in developing countries. [11,22] We reviewed the limited data on viruses seen in our patients and were unable to detect a specific indication with regard to adenovirus. The high incidence ofpulmonary TB (77.2%) and HIV infection (43.8%) makes preventive strategies for these illnesses imperative. The diseases often co-exist, increasing the risk for development of bronchiectasis.[6,23] Pulmonary TB produces bronchiectasis by bronchial obstruction secondary to an enlarged node or by distortion of segmental bronchi adjacent to a healing cavity.[24] In cases of bronchiectasis, co-infection with H. influenzae was most commonly a bacterial pathogen isolated from bronchoalveolar lavage, seen more frequently among HIV-infected children - similar to that seen in the literature.[2,6] Unilateral left lung involvement, especially the basal portion of the left lower lobe, was the most frequent site of disease. This could be due to the greater difficulty of drainage of the left main bronchi for the left lung and gravity-dependent retention of infected secretions.[24] Similar to other studies and based on HRCT scans, the usual morphological appearance of bronchiectasis in our cohort was the saccular type with atelectasis.[25]
Study limitations
The main limitation of the study is that it was performed at one centre. There are, however, very few cardiothoracic departments in SA and the study unit provides all cardiothoracic surgery services for 3 million children in KwaZulu-Natal. The retrospective nature of the study is also of concern, but the standardised electronic record-keeping system minimised the impact findings from lost data. Furthermore, it is recognised that the aetiology described is exclusively related to operative bronchiectasis. The lack of lung function results and long-term follow-up makes determining the improvement in quality of life difficult to assess objectively.
Conclusion
Although children from developing countries present late with advanced-stage bronchiectasis for surgery, pulmonary resection appears to be associated with a low morbidity and mortality. Pneumonectomy, to achieve complete resection for extensive disease, is a safe procedure, with minimal complications. Surgery in HIV-infected patients with bronchiectasis was not associated with worse outcomes than in non-HIV-infected patients and it is therefore not contraindicated. All bronchiectatic lobes or segments should be removed for optimum control of symptoms. The effective prevention and control of TB and HIV would be important strategies to reduce the burden of bronchiectasis among children in most developing countries, including SA.
We recommend that caregivers of children with bronchiectasis should be educated to report persistent symptoms early. Timely referral to a paediatric pulmonologist (if available) is strongly advised to define a specific aetiological diagnosis, institute adequate appropriate treatment and arrange close follow-up. Preventive measures, i.e. reducing exposure to risk factors such as smoking or chemoprophylaxis, would help to reduce progression. Referral to a cardiothoracic surgeon is essential for those in whom medical therapy is not successful.
Declaration. The research for this study was done in partial fulfilment of the requirements for MH's MMed (Cardiothoracic Surgery) degree at the University of KwaZulu-Natal, Durban, South Africa.
Acknowledgements. The authors thank the patients and medical staff at Inkosi Albert Luthuli Central Hospital for their participation in the study, and the administrative staff for permission to undertake this study.
Author contributions. JC, MH, PMJ: conceptualisation of the study; MH: data collection; PMJ, MH: analysis and write-up.
Funding. None.
Conflicts of interest. None.
References
1. Banjar H. Childhood bronchiectasis: A review. Bahrain Med Bull 2006;28(2):1-10. [ Links ]
2. Goyal V, Grimwood K, Marchant J, Masters IB, Chang AB. Pediatric bronchiectasis: No longer an orphan disease. Pediatr Pulmonol 2016;51(5):450-469. https://doi.org/10.1002/ppul.23380 [ Links ]
3. Sirmali M, Karasu S, Turut H, et al. Surgical management of bronchiectasis in childhood. Eur J Cardiothorac Surg 2006;31(1):120 -123. https://doi.org/10.1016/j.ejcts.2006.10.021 [ Links ]
4. Fujimoto T, Hillejan L, Stamatis G. Current strategy for surgical management of bronchiectasis. Ann Thorac Surg 2001;72(5):1711-1715. https://doi.org/10.1016/s0003-4975(01)03085-5 [ Links ]
5. Esme H, Eren S. Surgical treatment of bronchiectasis. Topics Thorac Surg 2012. https://doi.org/10.5772/26939 [ Links ]
6. Masekela R, Anderson R, Moodley T, et al. HIV-related bronchiectasis in children: An emerging spectre in high tuberculosis burden areas. Int J Tuberc Lung Dis 2012;16(1):114-119. https://doi.org/10.5588/ijtld.11.0244 [ Links ]
7. Lazarus JV, Olsen M, Ditiu L, Matias S. Tuberculosis-HIV coinfection: Policy and epidemiology in 25 countries in WHO European region. HIV Med 2008;9(6):406-414. https://doi.org/10.1111/j.1468-1293.2008.00567.x [ Links ]
8. Tasker AD, Flower CD. Imaging the airways: Hemoptysis, bronchiectasis, and small airways disease. Clinic Chest Med 1999;20(4):761-773. https://doi.org/10.1016/s0272-5231(05)70254-9 [ Links ]
9. Kim HY, Kwon JW, Seo J, et al. Bronchiectasis in children: 10-year experience at a single institution. Allerg Asthma Immunol Res 2011;3(1):39. https://doi.org/10.4168%2Faair.2011.3.1.39 [ Links ]
10. Brower KS, del Vecchio MT, Aronoff SC. The etiologies of non-cystic fibrosis bronchiectasis in childhood: A systematic review of 989 subjects. BMC Pediatr 2014;14(1):299. https://doi.org/10.1186/s12887-014-0299-y [ Links ]
11. Eastham KM, Fall AJ, Mitchell L, Spencer DA. The need to redefine non-cystic fibrosis bronchiectasis in childhood. Thorax 2004;59(4):324-327. https://doi.org/10.1136/thx.2003.011577 [ Links ]
12. Stephen T, Thankachen R, Madhu AP, Neelakantan N, Shukla V, Korula RJ. Surgical results in bronchiectasis: Analysis of 149 patients. Asian Cardiovasc Thorac Ann 2007;15(4):290-296. https://doi.org/10.1177%2F021849230701500405 [ Links ]
13. Otgun l, Karnak I, Tanyel FC, Senocak ME, Buyukpamukcu N. Surgical treatment of bronchiectasis in children. J Pediatr Surg 2004;39(10):15326. https://doi.org/10.1016/j.jpedsurg.2004.06.009 [ Links ]
14. Andrade CF, Melo IA, Holand AR, Silva ÉF, Fischer GB, Felicetii JC. Surgical treatment of non-cystic fibrosis bronchiectasis in Brazilian children. Pediatr Surg Int 2014;30(1):63-69. https://doi.org/10.1007/s00383-013-3420-7 [ Links ]
15. Haciibrahimoglu G, Fazlioglu M, Olcmen A, Gurses A, Bedirhan MA. Surgical management of childhood bronchiectasis due to infectious disease. J Thorac Cardiovasc Surg 2004;127(5):1361. https://doi.org/10.1016/j.jtcvs.2003.11.018 [ Links ]
16. Reed CE. Pneumonectomy for chronic infection: Fraught with danger? Ann Thorac Surg 1995;59(2):408-411. https://doi.org/10.1016/0003-4975(94)00867-7 [ Links ]
17. Ayed AK, Al-Rowayeh A. Lung resection in children for infectious pulmonary diseases. Pediatr Surg Int 2005;21(8):604-608. https://doi.org/10.1007/s00383-005-1485-7 [ Links ]
18. Kutlay H, Cangir AK, Enön S, et al. Surgical treatment in bronchiectasis: Analysis of 166 patients. Eur J Cardiothorac Surg 2002;21(4):634-637. https://doi.org/10.1016/S1010-7940(02)00053-2 [ Links ]
19. Le Roux B, Mohlala M, Odell J, Whitton I. A suppurative disease of the lung and pleural space, part II: Bronchiectasis. Curr Problem Surg 1986;23(2):159-194. https://doi.org/10.1016/0011-3840(86)90018-3 [ Links ]
20. Sanderson JM, Kennedy MC, Johnson MF, Manley DC. Bronchiectasis: Results of surgical and conservative management. A review of 393 cases. Thorax 1974;29(4):407-416. https://doi.org/10.1136/thx.29.4.407 [ Links ]
21. Pasteur MC, Bilton D, Hill AT. British Thoracic Society guideline for non-CF bronchiectasis. Thorax 2010;65(Suppl 1):i1-i58. https://doi.org/10.1136/thx.2010.136119 [ Links ]
22. Karadag BÜ, Karakoc F, Ersu R, Kut A, Bakac S, Dagli E. Non-cystic-fibrosis bronchiectasis in children: A persisting problem in developing countries. Respiration 2005;72(3):233-238. https://doi.org/10.1159/000085362 [ Links ]
23. Jeena PM, Mitha T, Bamber S, Wesley A, Coutsoudis A, Coovadia HM. Effects of the human immunodeficiency virus on tuberculosis in children. Tubercle Lung Dis 1996;77(5):437-443. https://doi.org/10.1016/s0962-8479(96)90117-3 [ Links ]
24. Deslauries J, Goulet S, Franc B. Surgical treatment of bronchiectasis and broncholithiasis. In: Franco LF, Putnam JB. Advanced Therapy in Thoracic Surgery. Hamilton, ON: B C Decker, 1998:300-309. [ Links ]
25. Habesoglu MA, Ugurlu AO, Eyuboglu FO. Clinical, radiologic, and functional evaluation of 304 patients with bronchiectasis. Ann Thorac Med 2011;6(3):131. https://doi.org/10.4103%2F1817-1737.82443 [ Links ]
 Correspondence:
Correspondence:
M Hbish
m-ihbesh@hotmail.com
Accepted 2 August 2021














