Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.16 no.2 Pretoria Jun. 2022
http://dx.doi.org/10.7196/sajch.2022.v16i2.1893
RESEARCH
The trajectory of general movements from birth until 12-14 weeks corrected age in very low-birthweight and extremely low-birthweight infants born preterm
R KrynauwI; J C F du PreezII; J I van ZylIII; M BurgerIV
IMSc Physiotherapy (Paediatric Neurology); Physiotherapy Division, Department of Health and Rehabilitation Science, Faculty of Medicine and Health Sciences, Stellenbosch University Cape Town, South Africa
IICert Neonatology; Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIIMB ChB; Department of Paediatrics and Child Health, Tygerb erg Hospital, Cape Town, South Africa
IVMSc Physiotherapy (Paediatric Neurology); Physiotherapy Division, Department of Health and Rehabilitation Science, Faculty of Medicine and Health Sciences, Stellenbosch University Cape Town, South Africa
ABSTRACT
BACKGROUND: General movement assessment (GMA) is an assessment tool with high predictive validity for neurodevelopmental outcomes in preterm infants. Information available describing the trajectory of general movements (GMs) in high-risk preterm-born infants and the use thereof in low- and middle-income countries is limited
OBJECTIVE: To describe the trajectories of GMs from birth until 12 - 14 weeks' corrected age, and determine the association of known perinatal risk factors on GM trajectories in very low-birthweight and extremely low-birthweight preterm infants
METHODS: This was a longitudinal, prospective cohort study with 119 preterm infants born at <33 weeks' gestation and with a birthweight <1 500 g. GMs were recorded at four key age periods: 1-2 weeks after birth to 33 weeks post menstrual age (PMA); 34 - 37 weeks PMA; term equivalent age (TEA); and 12 - 14 weeks corrected age. Detailed perinatal data were collected
RESULTS: A total of 300 GMAs were conducted, 157 during the preterm age, 55 during TEA and 88 at 12 - 14 weeks corrected age. At <33 weeks PMA, 96% of GMs were abnormal and 4% normal. At 34 - 37 weeks PMA, 89% of GMs were abnormal and 11% normal. All GMs recorded at term equivalent age were abnormal. At 12 - 14 weeks corrected age, 7% of GMs were abnormal and 93% normal
CONCLUSION: GMs were predominantly abnormal prior to term with a significant decrease in abnormality at 12 - 14 weeks corrected age. Lower birthweight and lower PMA were associated with increased odds for abnormal GMs. In a resource-constrained environment, observing GMs at 12 - 14 weeks corrected age (during the fidgety period) is a time- and cost-effective method to determine the risk for adverse neuro development
Significant advances in perinatal and neonatal care have improved survival rates for preterm infants. This increase in survival is mirrored by an increase in the risk of motor and cognitive impairment.[1] Early identification and intervention for infants at risk for neurodevelopmental disorders are associated with improved motor developmental outcomes during infancy (0 - <3 years), and enhanced cognitive function up to preschool age (3 - <5 years).[2] Among the motor impairments reported in preterm infants, cerebral palsy (CP) remains one of the most common,[3] with a higher prevalence of children with CP reported in Africa than the estimated 2 -2.5 cases per 1 000 live births reported in most studies conducted in the USA and Europe.[4,5] Unfortunately, the screening and identification of developmental disabilities in high-risk infants in Africa has been inadequate.[5] Low- and middle-income countries (LMICs) such as South Africa (SA) are unable to provide costly technical evaluation procedures needed to detect brain dysfunction in high-risk infants.[6]
In SA public hospitals, high-risk preterm infants are discharged when they have adequate weight gain and are medically stable, usually at 34 - 36 weeks post menstrual age (PMA) and weighing 1 800 g. These public hospitals have limited available beds and a high patient turnover. Mothers returning with their infants after discharge to impoverished rural areas often have inadequate access to follow-up medical care. As a result, premature infants who are at high risk for neurodevelopmental disorders are often lost to follow-up, medical management and effective interventions. Infants born preterm with undiagnosed CP are therefore at risk for secondary complications such as muscle/tendon contractures, bony torsion, hip displacement and spinal deformities.[7] Therefore, an inexpensive, reliable and non-invasive method for early identification of CP or other neurological disorders is warranted.
Prof. Heinz Prechtl and his co-workers developed such a method in the early 1990s. Prechtl's qualitative assessment of general movements (GMs) is an assessment tool that evaluates the quality of spontaneous movement patterns in infants. The nervous system of the fetus and young infant generates spontaneous movement patterns endogenously, i.e. without being triggered by specific sensory input.[8] From as young as 9 weeks PMA, generalised and very complex movements involving the whole body start to occur. These complex movement patterns are called GMs. They are age-specific, continue after birth and can be observed until 20 weeks post term age, when purposeful antigravity movements start to dominate.[8]
Before term age, GMs are called preterm or fetal movements, from term age until 6-9 weeks post term age they are called writhing movements and from 9 weeks until 20 weeks post term they are called fidgety movements.[8] Normal GMs are fluent and elegant and have a complex and variable character.[8] Periventricular brain lesions can lead to a disruption of the corticospinal projections and lead to abnormal GMs, which are movements characterised by a loss of complexity and variability. Abnormal GMs have a monotonous or poor repertoire, or are stiff and cramped, or chaotic.[8] A persistent pattern of cramped synchronised GMs and the absence of fidgety movements are highly predictive for the development of CP.[8] General movement assessments (GMAs) are quick and easy to perform, and are cost-effective compared with other investigations traditionally used, such as magnetic resonance imaging, brain ultrasound and traditional neurological examination.[8] Various systematic reviews have validated the qualitative assessment of GMs as a reliable predictor of CP.[9-11] Their straightforward and easy applicability makes GMAs an ideal tool for assessing the young nervous system, especially in low-resource settings.[6] Studies on GMAs in LMICs are rare,[6] with only one study conducted to date in SA, on GMAs at 12 - 15 weeks post term age.[9]
Although the qualitative assessments of GMs have been widely reported, most studies assessed GMs at 12 - 15 weeks post term age and term age, while only a few studies reported on GM trajectories during preterm age.[12-15] There is therefore a limited understanding of GM trajectories in preterm (32 - 36 weeks PMA), very preterm (28 - 31 weeks PMA) and extremely preterm (<28-week PMA) infants.[15] The high predictive validity of GMs relies on developmental trajectories, since a trajectory of GMAs is more accurate at predicting an infant's neurodevelopmental outcome than single assessments.[8,15] A systematic review found that children born preterm with consistently abnormal GMs up to 8 weeks after term had an intelligence quotient (IQ) of 5 - 13 points lower than that of children whose GMAs normalised after term age.[16] The early neurodevelopment of the preterm infant may be negatively influenced by perinatal factors such as intraventricular haemorrhage, necrotising enterocolitis, bronchopulmonary dysplasia and postnatal corticosteroids.[17,18] Knowledge and understanding of GM trajectories and the effect of adverse perinatal factors is essential to compare and analyse in future studies on the neurodevelopmental outcome of high-risk infants.
The primary aim of the present study was to assess the trajectory of GMs from preterm age until 12 - 14 weeks corrected age in very low-birthweight (VLBW) and extremely low-birth weight (ELBW) infants who were admitted to Tygerberg Children's Hospital (TCH) in Cape Town, SA. The objectives of the study were to describe the association between adverse perinatal factors and GM trajectories.
Methods
Study design and participants
A longitudinal, prospective cohort design with repeated measures was conducted. A successive sampling method was used to recruit preterm infants born before 33 weeks' gestation and weighing <1 500 g, between 1 December 2017 and 1 May 2018, and admitted to the neonatal wards or to the neonatal intensive care unit (NICU) at TCH in Cape Town, SA. The following exclusion criteria applied: infants diagnosed with congenital/chromosomal defects known to affect neurodevelopment (e.g. Down syndrome or Edwards syndrome); infants with birth malformations of the central nervous system (e.g. myelomeningocele); infants diagnosed with congenital disorders (e.g. arthrogryposis multiplex congenital osteogenesis imperfecta congenital); and infants with microcephaly (53rd percentile). The study was approved by the Human Research Ethics Committee of the Faculty of Health Science at Stellenbosch University (ref. no. S17/08/142). Written informed consent was obtained from the parents or legal guardians of the infants enrolled in the study.
Procedure for GMA
Serial GMs were recorded during the following key age periods: 1 - 2 weeks after birth to 33 weeks PMA; 34 - 37 weeks PMA; term equivalent age (TEA) (full term age (39 weeks 0 days - 40 weeks and 6 days PMA) or late term age (41 weeks 0 days - 41 weeks and 6 days PMA)); and 12 - 14 weeks corrected age (fidgety period). Prior to term, GMs were recorded while infants were inpatients at TCH or adjacent hospitals. At TEA, GMs were recorded at surrounding hospitals, or as an outpatient at TCH if the patient has been discharged home. GMs at 12 - 14 weeks corrected age were recorded during the infants' first outpatient follow-up visit at the neonatal high-risk clinic at TCH. Only infants with at least two recorded GMAs were included in the study.
GM assessments were performed using a standardised procedure. A light-sensitive, high-quality camera phone was used directly from above. During all assessments, infants were recorded in supine position and were lightly dressed (thin nappy and vest). Before term, infants were assessed in the crib or incubator, and were videoed for 5-10 minutes (depending on how long it took to observe a spontaneous movement sequence). Recordings made during the preterm age were taken during awake and asleep behavioural states of the infant.[8] At TEA and 12 - 14 weeks corrected age, the infants were placed on a unicolour mattress or on the examination bed and videoed for 5 minutes, and recordings were made with the infant in an active alert state, with the absence of crying/fussing.
Assessment and scoring of GMs
GMs were independently scored by at least three qualified assessors with advanced GM certification from the GM trust (http://general-movements-trust.info/5/home). Assessors were blinded to the neonatal history of the infants as well as their previous GMA scores to avoid the assessors being influenced. GMs were assessed using Prechtl's method on the qualitative assessment of GMs.[19] From 1-2 weeks after birth (preterm age) until TEA, GMs were scored as follows:
Normal GMs: these movements are characterised by fluency and elegance, involving the whole body. They consist of variable patterns of flexion, extension and rotation of the limbs and rotation of the trunk, and are complex in nature.
Abnormal GMs: these were categorised as:
• poor repertoire: movements that are lacking complexity and speed, amplitude and force, often observed as slower than normal GMs. Movements tend to be repetitive and monotonous.
• cramped-synchronised: these movements are rigid in appearance, involving an almost simultaneous contraction and subsequent relaxation of all limbs and trunk muscles.
• chaotic: movements that are large and abrupt in nature, involving all limbs and lacking fluency and elegance.
At 12 - 14 weeks' corrected age (fidgety period), GMs were scored as follows:
• normal fidgety movements: characterised by small amplitude, moderate speed and variable acceleration of the trunk and limbs in all directions.
• abnormal fidgety movements: these look like normal fidgety movements, but their amplitude, speed and jerkiness are moderately or greatly exaggerated.
• absent fidgety movements: fidgety movements are not observed, but other movements like wiggling-oscillating arm movements, swiping movements of the arms and kicking of the legs can still be observed.
Credibility of analysis
Individual scores were compared within the group. In the case of score discrepancies, Prof. Christa Einspieler, a licensed senior GM Trust tutor, made the final decision.
Perinatal data
Perinatal information from the medical histories and neonatal course of the participating infants was collected. The data included: gestational age; birthweight; gender; ventilation and/or oxygen requirements; the presence of intraventricular haemorrhage or periventricular leukomalacia; necrotising enterocolitis, postnatal infections and HIV exposure.
Statistical analysis
Stata version 14 (StataCorp., USA) and SPSS version 24 (IBM Corp., USA) were used to analyse data. A p-value <0.05 was considered statistically significant. The proportion of infants with normal and abnormal GMs over time was reported at each of the four key time points, along with 95% confidence intervals (CIs). The change from one time point to the next in abnormal GMs was assessed by cross-tabulation of normal and abnormal GMs at adjacent time points, and also from the first time point to the last time point. McNemar's X2 test was used to assess statistical significance in the change in proportions between two key time points.
Logistic regression analysis adjusting for within-patient clustering over time was used to estimate the odds ratios and 95% CIs for the effects of time and the various confounding variables for the outcome of abnormal v. normal GMs. The potential confounders included were gestational age, birthweight, gender, type and duration of ventilation, total duration of oxygen via nasal cannula, length of hospitalisation, intraventricular haemorrhage grade III/IV, periventricular leukomalacia grade III/ IV, surgical necrotising enterocolitis, postnatal corticosteroids, small for gestational age, any surgical procedure, culture Gram-positive or negative sepsis, meningitis, exposure to HIV and multiple births.
Results
Demographic profile and characteristics of the study population
A total of 119 eligible infants were included in the study. During the course of the study, 12 infants passed away, of whom 9 were male. The majority of included infants were female (53%). The demographic profile and characteristics of the cohort are summarised in Table 1. A total of 300 GMAs were conducted: 110 at <33 weeks PMA, 47 at 34 - 37 weeks PMA, 55 at term age and 88 at 12 - 14 weeks post term age. A flowchart of the study participants and conduction of GMAs is presented in Fig. 1.

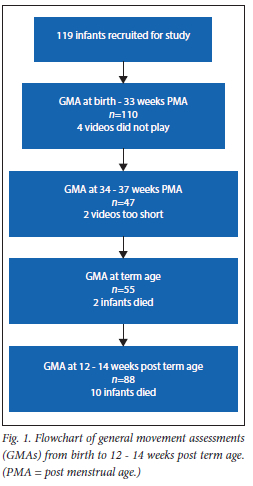
Results of GMAs
The GMA score results of the four different key assessment points are illustrated in Table 2. During the first time point (from birth to 33 weeks PMA), 110 infants were assessed, and at the second time point (34 - 37 weeks PMA), 47 infants. At TEA (the third time point) 55 infants were assessed, and during the final time point (12 -14 weeks corrected age), 88 GMAs were conducted.

Association between perinatal factors and GM outcomes
The association between perinatal risk factors listed in Table 1 and GMs were assessed using a logistic regression model. On univariate analysis, lower birthweight (p=0.043), gestational age at birth (p)=0.017), intraventricular haemorrhage grade IV (p<0.001) and time since birth (PMA in weeks) (p<0.001) were associated with increased odds for abnormal GMs. These findings are illustrated in Table 3.

Lower birthweight, gestational age at birth, intraventricular haemorrhage grade IV, and time since birth (PMA in weeks) were included in a multivariable analysis. Since birthweight and gestational age were highly correlated and thus collinear, gestational age was dropped from the final model.
Birthweight (p=0.046) and time (PMA in weeks) (p<0.001) were the only variables that remained significantly associated with abnormal GMs after adjustment for confounding variables listed in Table 1.
Discussion
The quality of spontaneous movement patterns observed in infants reflects the integrity of the young nervous system and serves as a predictor for later neurological outcomes.[8] Although the assessment of GMs has been utilised for over 25 years, to date, only six studies globally have reported on preterm and post-term GMA trajectories at the following key time points: preterm, term age (37 - 42 weeks PMA) and 12 - 15 weeks post term age.[20-25] The present study is the first such study conducted in Africa to describe the quality of GM trajectories in infants born before 33 weeks' gestational age till 12 - 14 weeks corrected age in VLBW and ELBW infants. The findings of the current study demonstrate that the majority of infants displayed abnormal GMs during preterm assessments. These results are consistent with the findings from previous studies that reported on at least two GMAs prior to term age in VLBW and ELBW infants.[12-15]
In the present study, no infants displayed normal GMs at term age. This increased proportion of abnormal GMs from preterm to term age differs from what was found in previous studies that reported on GMs from preterm to term age.[12,15,24,26] A possible explanation may be that, in the current study, the birthweight of 85% of the infants assessed at term age was <1 200 g. Furthermore, 73% of infants had a gestational age of <29 weeks. Both a lower birthweight and gestational age have been significantly associated with abnormal GMs at term age.[27] Another reason for not observing any normal GMs at term age is the difficulty with follow-up of infants who were discharged (see limitations). Most infants with recorded GMs at term were still admitted at term age. This likely reflects the extent of neonatal problems encountered by term-age infants who were still hospitalised. They would therefore be a high-risk cohort for neurodevelopmental disorders or other health-related disorders.
Our finding that none of the infants in the current study displayed abnormal fidgety movements at 12 - 14 weeks corrected age is similar to findings by other researchers.[28,30] Abnormal fidgety movements are extremely rare and of low predictive value.[19,31] In the current study, 7% of infants had absent fidgety movements at 12 -14 weeks corrected age, which is lower than the 9% reported in a previous study conducted at TCH on 115 VLBW preterm infants.[7] Other studies that included both high- and low-risk infants reported higher percentages of infants with absent fidgety movements.[28-30,32,33]
The significant decrease in the proportion of infants who displayed abnormal GMs from the first GMA (n=73) to the final GMA (n=5) is consistent with previous published findings.[22,24,26] GMs assessed during the fidgety period have a higher yield and are more feasible in a resource-constrained setting.
Influence of perinatal variables on GMs
Multivariable analysis of the association between certain perinatal risk factors and GM trajectories identified that an increase in birthweight and time (indicated as PMA in weeks) was inversely associated with an abnormal GM trajectory. This differs from what was reported in other studies.[15,26] Olsen et al.[15] reported infection as an independent variable associated with an increased risk for abnormal GMs. Zahed-Cheick et al.[26] found in a group of extremely preterm infants that gestational age at birth, nosocomial infections, chronic lung disease and patent ductus arteriosus were associated with abnormal preterm GMs. However, at 12 - 14 weeks corrected age, only gestational age at birth was correlated with absent fidgety movements, while no correlation with birthweight was found.[26]
The results of the current study are consistent with the findings of a recent study[27] that reported a lower birthweight to be associated with abnormal GMs at term age and 12 - 14 weeks corrected age. To the best of our knowledge, our study is the largest to date to report on GM trajectories measured at four key time points. This might explain why the study is the first to report on time (PMA in weeks) as a significant variable associated with GM outcome. De Vries et al.[13] recorded serial GMs during the first 10 days of life in very preterm and extremely preterm infants. They found that abnormal GMs were significantly related to preterm age. The younger the infants (PMA), the more often they presented with abnormal GMs. They concluded that an improvement in GM trajectories during the first week occurred in infants who had a higher birthweight and gestational age.
The present study is unique as 23% (n=27) of the cohort were HIV-exposed but uninfected. On univariable analysis, HIV exposure during pregnancy was not significantly associated with an abnormal GM trajectory. The quality of GM trajectories and neurological outcome in HIV-exposed but uninfected as well as exposed and infected children is a largely under-researched field. Only one previous study has reported on GMs in a HIV-exposed cohort.[34] The authors found that comorbid HIV and maternal opiate exposure were associated with an abnormal GM trajectory from term age till 5 months post term age, and that infants with HIV infection did not differ from HIV-exposed but uninfected infants with respect to their GM quality. A large prospective study found that maternal opioid use is associated with inadequate antenatal care and a higher likelihood of poor nutrition and polysubstance use, including alcohol, cigarettes, marijuana and stimulants. Consequently prenatal opioid exposure was associated with poor birth outcomes and adverse childhood physical health and neurodevelopmental outcomes.[35] A systematic review found that once confounders such as maternal substance misuse were accounted for, studies did not demonstrate developmental delays in HIV-exposed, uninfected infants up to the age of 2 years.[36] Since most of the evidence came from high-income countries, the researchers suggested that other factors such as poverty and early infant malnutrition and growth in low-resource settings may affect neuro development of HIV-exposed, uninfected infants.[36]
Previous studies have reported intraventricular haemorrhage (IVH) grade III and IV to be associated with abnormal GM trajectories in preterm infants.[15,33] The small sample size of infants in the present study diagnosed with IVH grade III and IV may explain why IVH was not significantly associated with abnormal trajectories. Although sepsis was not significantly associated with abnormal GMs, previous studies[15,26] have reported an association between post-natal infections and abnormal GMs. Evidence on the significance of infection on GM outcome remains conflicting
Study limitations
The main limitation of the study was the significant decrease in the number of GMAs done from the first assessment (birth to 33 weeks PMA) (n=110) to the second assessment (34 - 37 weeks PMA) (n=47) and term age assessments (n=55). At TCH, once medically stable, infants are transferred to district hospitals and other lower care facilities, or discharged home. Owing to the fact that most infants were discharged from hospital before the second assessment, follow-up appointments had to be arranged. During the course of the study, the City of Cape Town was plagued by major bus and taxi strikes as well as protest actions. Since most of the parents/ guardians made use of public transport and their safety could not be guaranteed, a large proportion of infants was unable to attend their preterm and term age follow-up assessments. Public transport is also costly, and given that most of the population comes from a lower socioeconomic background, many parents were unable to bring their infants in for a term age GMA. Parents of discharged infants who were not able to attend follow-up appointments were asked to send a video recording of their infant via WhatsApp, and were compensated for their data usage. However, not all parents had access to smartphones and WhatsApp, or the cellphone recordings were of such low quality that it was not possible to assess the video recording of GMs. A large number of infants with 34 - 37 weeks PMA and term GMAs were still hospitalised. Since these infants had a more complicated medical history, this might explain the reduced number of normal GMs observed at 34 - 37 weeks PMA, as well as the absence of normal GMs at term age.
Notwithstanding the setback of follow-up at preterm and term age, 88 infants were assessed at 12 - 14 weeks corrected age, and a total of 300 assessments were conducted over the four key time periods.
In the present study, individual infant trajectories were not described. Individual trajectories, especially for infants displaying temporary normal or cramped-synchronised GMs prior to term or at term age, may provide a better understanding of the relationship between perinatal risk factors and GM quality. Furthermore, infants were only assessed until 12 - 14 weeks corrected age. Although it was not part of the scope of the current study, neurological assessments conducted at 12 and 24 months corrected age may be of value to describe the effect of GM trajectories and perinatal risk factors on long-term neurodevelopmental outcomes.
Clinical and research implications
Heinz Prechtl and his colleagues encouraged the use of serial assessments to provide a comprehensive portrayal of the infants neurodevelopmental trajectory.[19] However, the results of the present study indicate that assessment of preterm and term GM trajectories does not necessarily enable earlier identification of infants at risk for neurodevelopmental difficulties in our study population. In a low-resource setting, it is therefore not clinically useful to allocate time and resources to conduct preterm and term age GMs, as they are likely to be abnormal and transition to normal over time. High-risk preterm infants in low- and medium-resource settings should rather be assessed at 12 - 14 weeks corrected age, as has previously proven to be of high predictive value at TCH.[7] Furthermore, infants with a lower birthweight should be targeted for more frequent follow-up, as they remain the highest risk group for neurological deficits.
The study cohort will be followed up to determine the relationship between GM trajectories and long-term neuro development. Future research should describe individual infant trajectories together with long-term neurological follow-up in order to establish the influence of perinatal factors on long-term outcome.
Conclusion
Using trajectories of GMs is a novel way of tracking the integrity of the developing neurological system. In resource- and time-constrained settings such as SA, it is important to evaluate the feasibility of such an approach. Our study demonstrates that most GMs have normalised by the fidgety movement period, and it is therefore more feasible for the group (but not the individual infant) to do GMAs at 12 - 15 weeks post term. Lower birthweight and lower PMA (time) were associated with increased odds for abnormal GMs. Infants with a lower birthweight should be targeted for early (at 12 - 14 weeks corrected age) and frequent follow-up as they remain the most at-risk group for neurological deficits.
Declaration. This research was submitted in partial fulfilment of the requirements or the degree of Masters of Physiotherapy in the Faculty of Medicine and Health Sciences at Stellenbosch University(Paed).
Acknowledgements. The authors thank Dr Tonya Esterhuizen, a biostatistics consultant within the Division of Epidemiology and Biostatistics, Stellenbosch University for assisting with the design and analysis of this study through support from the Faculty of Medicine and Health Sciences deans fund. The authors would also like to thank Jacqui Cooper (paediatric occupational therapist and qualified GM assessor) and Adri Crafford (paediatric physiotherapist and qualified GM assessor) who assisted MB, JCFdP and JIvZ with the GMAs.
Author contributions. RK was the main author and was responsible for data collection. RK, MB, JCFdP and JIvZ were responsible for the conceptualisation of the study design. MB, JCFdP and JIvZ were responsible for the GMAs. RK, MB, JCFdP and JIvZ were responsible for data analysis and interpretation. RK was responsible for writing and MB, JCFdP and JIvZ for editing of the manuscript.
Funding. During the completion of this manuscript, MB was funded by the SA Medical Research Council through its Division of Research Capacity Development under the National Health Scholarship Programme from funding received from the Public Health Enhancement Fund/SA National Department of Health.
Conflicts of interest. None.
References
1. Lester BM, Marsit CJ, Giarraputo J, Hawes K, LaGasse LL, Padbury JF. Neurobehavior related to epigenetic differences in preterm infants. Epigenomics 2015;7(7):1123-1136. https://doi.org/10.2217/epi.15.63 [ Links ]
2. Spittle A, Orton J, Anderson PJ, Boyd R, Doyle LW. Early developmental intervention programmes provided post hospital discharge to prevent motor and cognitive impairment in preterm infants. Cochrane Database Syst Rev 2015;11:CD005495. https://doi.org/10.1002/14651858.cd005495.pub4 [ Links ]
3. Spittle AJ, Orton J. Cerebral palsy and developmental coordination disorder in children born preterm. Semin Fetal Neonat M 2014;19(2):84-89. https://doi.org/10.1016/j.siny.2013.11.005 [ Links ]
4. Gottlieb CA, Maenner MJ, Cappa C, Durkin MS. Child disability screening, nutrition, and early learning in 18 countries with low and middle incomes: Data from the third round of UNICEF's Multiple Indicator Cluster Survey (2005 - 2006). Lancet 2009;374(9704):1831-1839. https://doi.org/10.1016/s0140-6736(09)61871-7 [ Links ]
5. Donald KA, Samia P, Kakooza-Mwesige A, Bearden D. Pediatric cerebral palsy in Africa: A systematic review. Semin Pediatr Neurol 2014;21(1):30-35. https://doi.org/10.1016/j.spen.2014.01.001 [ Links ]
6. Tomantschger I,Herrero D,Einspieler C,Hamamura C,VoosMC,MarschikPB. The general movement assessment in non-European low-and middle-income countries. Revista de Saude Publica 2018;52:6. https://doi.org/10.11606/sl518-8787.2018052000332 [ Links ]
7. Burger M, Frieg A, Louw QA. General movements as a predictive tool of the neurological outcome in very low and extremely low birthweight infants - a South African perspective. Early Hum Dev 2011;87(4):303-308. https://doi.org/10.1016/j.earlhumdev.2011.01.034 [ Links ]
8. Einspieler C, Prechtl HF. Prechtl's assessment of general movements: A diagnostic tool for the functional assessment of the young nervous system. Dev Disabil Res 2005;11(1):61-67. https://doi.org/10.1002/mrdd.20051 [ Links ]
9. Burger M, Louw QA. The predictive validity of general movements - a systematic review. Eur J Paediatr Neurol 2009;13(5):408-420. https://doi.org/10.1016/j.ejpn.2008.09.004 [ Links ]
10. Darsaklis V, Snider LM, Majnemer A, Mazer B. Predictive validity of Prechtl's method on the qualitative assessment of general movements: A systematic review of the evidence. Dev Med Child Neurol 2011;53(10):896-906. https://doi.org/10.1111/j.1469-8749.2011.04017.x [ Links ]
11. NovakI, Morgan C, Adde L, et al. Early, accurate diagnosis and early intervention in cerebral palsy: Advances in diagnosis and treatment. JAMA Pediatr 2017;171(9):897-907. https://doi.org/10.1001/jamapediatrics.2017.1689 [ Links ]
12. Bos AF, van Loon AJ, Hadders-Algra M, Martijn A, Okken A, Prechtl HF. Spontaneous motility in preterm, small-for-gestational age infants II. Qualitative aspects. Early Hum Dev 1997;50(1): 131-147. https://doi.org/10.1016/s0378-3782(97)00098-4 [ Links ]
13. De Vries NK, Erwich JJ, Bos AF. General movements in the first fourteen days of life in extremely low birthweight (ELBW) infants. Early Hum Dev 2008;84(11):763-768. https://doi.Org/10.1016/j.earlhumdev.2008.05.003 [ Links ]
14. De Vries NK, Bos AF. The quality of general movements in the first ten days of life in preterm infants. Early Hum Dev 2010;86(4):225-229. https://doi.org/10.1016/j.earlhumdev.2010.03.004 [ Links ]
15. Olsen JE, Brown NC, Eeles AL, et al. Trajectories of general movements from birth to term-equivalent age in infants born <30 weeks' gestation. Early Hum Dev 2015;91(12):683-688. https://doi.org/10.1016/j.earlhumdev.2015.09.009 [ Links ]
16. Einspieler C, Bos AF, Libertus ME, Marschik PB. The general movement assessment helps us to identify preterm infants at risk for cognitive dysfunction. Front Psychol 2016;7:406. https://doi.org/10.3389/fpsyg.2016.00406 [ Links ]
17. Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for cerebral palsy and motor impairment in children born very preterm or very low birthweight: A systematic review. Dev Med Child Neurol 2016;58(6):554-569. https://doi.org/10.1111/dmcn.12972 [ Links ]
18. Xiong T, Gonzalez F, Mu DZ. An overview of risk factors for poor neurodevelopmental outcome associated with prematurity. World J Pediatr 2012;8(4):293-300. https://doi.org/10.1007/s12519-012-0372-2 [ Links ]
19. Einspieler C, Prechtl HFR, Bos AF, Ferrari F, Cioni G (editors). Prechtfis Method on the Qualitative Assessment of General Movements in Preterm, Term and Young infants. London: Mac Keith Press, 2004. [ Links ]
20. Cioni G, Ferrari F, Einspieler C, Paolicelli PB, Barbani T, Prechtl HF Comparison between observation of spontaneous movements and neurologic examination in preterm infants. J Pediatr 1997;130(5):704-711. https://doi.org/10.1016/s0022-3476(97)80010-8 [ Links ]
21. Bos AF, van Asperen RM, de Leeuw DM, Prechtl HF. The influence of septicaemia on spontaneous motility in preterm infants. Early Hum Dev 1997;50(1):61-70. https://doi.org/10.1016/s0378-3782(97)00093-5 [ Links ]
22. Bos AF, Martijn A, van Asperen RM, Hadders-Algra M, Okken A, Prechtl HF. Qualitative assessment of general movements in high-risk preterm infants with chronic lung disease requiring dexamethasone therapy. J Pediatr 1998;132(2):300-306. https://doi.org/10.1016/s0022-3476(98)70449-4 [ Links ]
23. Ferrari F, Cioni G, Einspieler C, et al. Cramped synchronised general movements in preterm infants as an early marker for cerebral palsy. Arch Pediat AdolMed2002;156(5):460-467. https://doi.org/10.1001/archpedi.156.5.460 [ Links ]
24. Garcia JM, Gherpelli JL, Leone CR. The role of spontaneous general movement assessment in the neurological outcome of cerebral lesions in preterm infants. J Pediatr (Rio J) 2004;80:296-304. [ Links ]
25. Nakajima Y, Einspieler C, Marschik PB, Bos AF, Prechtl HF. Does a detailed assessment of poor repertoire general movements help to identify those infants who will develop normally? Early Hum Dev 2006;82(1):53-59. https://doi.org/10.1016/j.earlhumdev.2005.07.010 [ Links ]
26. Zahed-Cheikh M, Brevaut-Malary V, Busuttil M, Monnier AS, Roussel M, Gire C. Comparative analysis of perinatal and postnatal factors, and general movement in extremely preterm infants. Brain Dev 2011;33(8):656-665. https://doi.Org/10.1016/j.braindev.2010.10.023 [ Links ]
27. Ma L, Meng LD, Chen YH, Yi MJ, Wang JW, Cao AH. [Risk factors associated with general movement quality in infants], HK J Paediatr 2018;23(3):225-232. [ Links ]
28. Ivanov IS, Shukerski KG, Chepisheva EV. Spontaneous motor activity three months after birth in comparison with clinical and ultrasound studies. Folia Medica 2005;47(2):18-23. [ Links ]
29. Adde L, Rygg M, Lossius K, 0berg GK, Stoen R. General movement assessment: Predicting cerebral palsy in clinical practice. Early Hum Dev2007;83(l):13-18. https://doi.Org/10.1016/j.earlhumdev.2006.03.005 [ Links ]
30. Sharp M, Coenen A, Amery N. General movement assessment and motor optimality score in extremely preterm infants. Early Hum Dev 2018;124:38-41. https://doi.Org/10.1016/j.earlhumdev.2018.08.006 [ Links ]
31. Prechtl HF, Einspieler C, Cioni G, Bos AF, Ferrari F, Sontheimer D. An early marker for neurological deficits after perinatal brain lesions. Lancet 1997;349(9062):1361-1363. https://doi.org/10.1016/s0140-6736(96)10182-3 [ Links ]
32. Spittle AJ, Spencer-Smith MM, Cheong JL, et al. General movements in very preterm children and neurodevelopment at 2 and 4 years. Pediatrics 2013;132(2):452-458. https://doi.org/10.1542/peds.2013-0177 [ Links ]
33. Spittle AJ, Brown NC, Doyle LW, et al. Quality of general movements is related to white matter pathology in very preterm infants. Pediatrics 2008; 121 (5) :1184-1189. https://doi.org/10.1542/peds.2007-1924 [ Links ]
34. Palchik AB, Einspieler C, Evstafeyeva IV, Talisa VB, Marschik PB. Intra-uterine exposure to maternal opiate abuse and HIV: The impact on the developing nervous system. Early Hum Dev 2013;89(4):229-235. https://doi.org/10.1016/j.earlhumdev.2013.02.004 [ Links ]
35. Azuine RE, Ji Y, Chang HY, et al. Prenatal risk factors and perinatal and postnatal outcomes associated with maternal opioid exposure in an urban, low-income, multiethnic US population. JAMA NetworkOpen 2019;2(6):e196405. https://doi.org/10.1001/jamanetworkopen.2019.6405 [ Links ]
36. Le Doare K, Bland R, Newell ML. Neurodevelopment in children born to HIV-infected mothers by infection and treatment status. Pediatrics 2012;130(5):e1326-1344. https://doi.org/10.1542/peds.2012-0405 [ Links ]
 Correspondence:
Correspondence:
R Krynauw
rezevanzyl@gmail.com
Accepted 10 August 2021














