Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.16 no.2 Pretoria Jun. 2022
http://dx.doi.org/10.7196/sajch.2022.v16i2.1816
RESEARCH
Paediatric gastrointestinal endoscopy: Experience in Red Cross War Memorial Children's Hospital, Cape Town, South Africa
C B EkeI, II, III; R A BrownIV; R J de LacyV, VI; E A GoddardVII, VIII
IM Phil; Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, South Africa
IIM Phil; Division of Paediatric Gastroenterology, Department of Paediatrics and Child Health, Red Cross War Memorial Children's Hospital Cape Town, South Africa
IIIM Phil; Department of Paediatrics, College of Medicine, University of Nigeria, Enugu, Nigeria
IVM Phil; Department of Paediatric Surgery, Red Cross War Memorial Children's Hospital, Cape Town, South Africa
VFC Paed (SA); Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, South Africa
VIFC Paed (SA); Division of Paediatric Gastroenterology, Department of Paediatrics and Child Health, Red Cross War Memorial Children's Hospital Cape Town, South Africa
VIIMB ChB, PhD; Department of Paediatrics and Child Health, Faculty of Health Sciences, University of Cape Town, South Africa
VIIIMB ChB, PhD; Division of Paediatric Gastroenterology, Department of Paediatrics and Child Health, Red Cross War Memorial Children's Hospital Cape Town, South Africa
ABSTRACT
BACKGROUND: Endoscopy is an important diagnostic and therapeutic mode of management in children with gastrointestinal disorders
OBJECTIVE: To determine the indications, endoscopic yields and impact of the service on the ongoing health and complications among children who underwent gastrointestinal endoscopy at Red Cross War Memorial Children's Hospital, Cape Town
METHODS: A 10-year (2007 - 2016) retrospective study of children <18 years old who underwent gastrointestinal endoscopy was undertaken using relevant patients' variables obtained from their hospital medical records. Data were analysed using Stata 13.1 (p<0.05
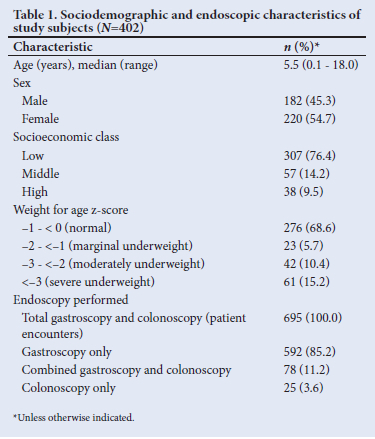
RESULTS: A total of 402 children underwent a total of 695 gastrointestinal endoscopic procedures: 592 (85.2%) were gastroscopies, 78 (11.2%) combined gastroscopies with colonoscopies and 25 (3.6%) colonoscopy-only procedures, respectively. The main diagnostic indications for gastroscopy, gastroscopy combined with colonoscopy and colonoscopy-only were chronic abdominal pain (n=49; 12.2%), suspected inflammatory bowel disease (n=30; 7.5%) and rectal bleeding (n=13; 52.0%) respectively. The most common therapeutic indication for gastroscopy was change of a percutaneous endoscopic gastrostomy (n=143; 35.6%) while for colonoscopy 6 (5.8%) had polypectomy. Abnormal histopathological results were made from both macroscopically normal- and abnormal-looking tissues, though with no statistically significant relationship
CONCLUSION: Endoscopy offers diagnostic and therapeutic options in children. Positive histological findings were obtained in some cases where gastrointestinal mucosae appeared normal. There is need to obtain biopsies from both macroscopically normal- and abnormal-looking gastrointestinal mucosae as positive histological findings could be made from them and hence improve diagnostic yield
Endoscopy has evolved to become an invaluable tool in the diagnosis and therapy of a variety of gastrointestinal disorders[1,2] in children, owing to the technological advancements in endoscopy designs and its devices.[3,4]
Improvements in sedation, anaesthesia[4] equipment and monitoring of vital signs of patients[5] during endoscopic procedures have added to the increased and safe use of gastrointestinal endoscopy in children, particularly younger infants and neonates.[5,7]
Indications for gastrointestinal endoscopy are diverse and fundamental to the assessment, treatment and follow-up/surveillance of children with gastrointestinal disorders, providing high diagnostic and therapeutic yields.[8]
Histopathological examination of tissue biopsies obtained from both macroscopically normal- and abnormal-looking tissues at endoscopy has improved the diagnosis of some gastrointestinal diseases.[9]
The sensitivity of endoscopic examinations varies with the age of the child and indication for the oesophagogastroduodenoscopy (OGD) and colonoscopy procedures, respectively.[10,11] Generally gastrointestinal endoscopy has stood out as an accurate and informative method of assessing upper and lower gastrointestinal disorders, and endoscopic procedures should therefore be performed only in clinical conditions in which they have shown superiority over other diagnostic methods, including gastrointestinal contrast studies, X-rays and ultrasonography, among others.[8,12]
Various expert groups and organisations including the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) and European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) as well as the European Society of Gastrointestinal Endoscopy (ESGE),[12,13] and experts in the field,[14] have assessed the different guidelines for the use of gastrointestinal endoscopy in children, and recommended that there should be clear indications for undertaking an endoscopic procedure to ensure that its findings impact on patient management.
There is a paucity of data on paediatric gastrointestinal endoscopy in sub-Saharan Africa, including South Africa (SA), with most studies jointly reported by paediatricians in conjunction with adult gastroenterologists.[15-17]
The current study is a 10-year clinical audit of the paediatric gastrointestinal endoscopies conducted by paediatric gastroenterologists and trainee fellows under supervision in a paediatric specialist tertiary centre, Red Cross War Memorial Children's Hospital (RCWMCH), Cape Town, SA.
Although several published guidelines exist on paediatric gastrointestinal endoscopy from developed countries, it is envisaged that considering the long-standing experience of continuous gastrointestinal endoscopy programme in the centre, its findings will be more adaptable to most resource-poor/limited settings, particularly in sub-Saharan Africa.
Such findings will help paediatric gastroenterologist(s) and by extension paediatricians, with interests in endoscopy services to expand and improve on the quality as well as outcome of their gastrointestinal endoscopy services.
The objectives of the study were to assess the presenting symptoms, indications, histological yields, impact on management and complications among children and adolescents who underwent medical gastrointestinal endoscopy at RCWMCH, Cape Town.
Method
Study setting
This study was conducted at RCWMCH, which is a tertiary paediatric hospital affiliated to the University of Cape Town, SA.
All gastrointestinal endoscopies in children in the hospital were done following standard protocols by either consultant paediatric gastroenterologist(s) or trainee paediatric gastroenterology fellows under supervision. All gastrointestinal endoscopies in the unit were done under general anaesthesia administered by anaesthetists. RCWMCH only treats patients up to 13 years of age unless they have a chronic illness, and then they are followed up until 18 years of age.
At RCWMCH, cases of foreign body or caustic ingestions, oesophageal dilatations and laparoscopic percutaneous endoscopic gastrostomy (PEG) insertion were undertaken by the paediatric surgical team on separate endoscopy lists, and did not form part of this review.
Study design
This was a retrospective cross-sectional descriptive study undertaken among children and adolescents who underwent upper and lower gastrointestinal endoscopies performed by paediatric medical gastroenterologists from 1 January 2007 to 31 December 2016. This study did not include procedures performed by the paediatric surgeons in the centre.
Ethical approval
Study ethical approval was obtained from the University of Cape Town Human Research Ethics Committee (ref. no. 089/2017), while written permission was obtained from the RCWMCH research committee and management prior to the commencement of the study.
Inclusion criteria
All children who had an OGD and/or colonoscopy with complete medical records were included in the study.
Exclusion criteria
Patients who underwent gastrointestinal endoscopy but with incomplete medical records during the period under review were excluded.
Procedure
All gastrointestinal endoscopies were performed under general anaesthesia. At gastroscopy (OGD), multiple tissue biopsies were taken from the oesophagus, stomach and duodenum, even if the tissue macroscopically looked normal.
All colonoscopy biopsies were taken from multiple sites in the colon. The patients were usually admitted a day before the procedure, during which time they underwent standard bowel preparation using polyethylene glycol (GoLytely; PEG) at a dose of 80 mL/kg body weight, usually starting from ~13h00 on the day before the procedure, and given only a soft lunch and no supper. The PEG is usually given orally, and in infants and younger children who cannot drink effectively, it is given via a nasogastric tube in graded doses until the bowel is clear.
Study datasheet
Information retrieved for each patient included sociodemographic characteristics, initial presenting symptoms, type of gastrointestinal endoscopy performed (gastroscopy, combined gastroscopy with colonoscopy or colonoscopy only) with specific indication(s), macroscopic findings on endoscopy, complication(s) following endoscopy, histological diagnosis and impact on management following the endoscopic procedure.
The data were collected from the hospital's medical records as well as the paediatric gastroenterology unit and endoscopy and histopathology databases, and captured on a study datasheet.
Diagnostic characteristics
Diagnostic yield of endoscopy in the current study was classified as either positive (presence of any macroscopic endoscopy and/or histological abnormality found, excluding mild inflammation on histology) or negative (no or minor abnormality/normal histology) effecting a positive contribution.[18,19]
Mild inflammation was not regarded as a positive histological outcome as the clinical significance of isolated mild histological findings is inconclusive.[18,19]
Endoscopy diagnostic yield was calculated for initial examination involving diagnostic indications for upper and lower endoscopy, respectively.
Socioeconomic class determination
Patients were classified as low (HO or HI), middle (H2) or high (H3) socioeconomic class (SEC) according to their gross income per annum for the purposes of service fee determination, according to the uniform fee schedule regulations for healthcare services rendered by the Western Cape Province, Department of Health, SA, 2017.[20]
Data analysis
Data analysis was done using Stata 13.1 (Stata Corp, USA). Categorical variables were presented as frequency tables and charts, while numerical variables were presented as descriptive measures, expressed as median and range.
The association between categorical variables was assessed using the Pearson x2 test or Student's f-test where appropriate. A p-value <0.05 was considered statistically significant.
Results
Characteristics of study participants
A total of 402 patients with complete medical records were studied. There were 220 (54.7%) girls, with a female-to-male ratio of 1:0.8. Their median age was 5.5 (range: 0.1 - 18) years, 394 (98.0%) were <13 years old and of normal weight (n=276; 68.6%), and most were of low SEC (n=307; 76.4%) (Table 1).
Endoscopic procedures
Of the total 695 gastrointestinal endoscopic procedures, 592 (85.2%) were gastroscopies, 78 (11.2 %) gastroscopies combined with colonoscopies and 25 (3.6%) colonoscopy only (Table 1). The median numbers of gastroscopies and colonoscopies performed per patient were 1 (range 1 - 12) and 2(1- 4), respectively
Presenting symptoms
The presenting symptoms for gastroscopy, combined gastroscopy with colonoscopy and colonoscopy only were as shown in Figs 1, 2 and 3, respectively.


The most common presenting symptoms in children undergoing gastroscopy (as shown in Fig. 1) were patients evaluated for PEG insertion for feeding (therapeutic gastroscopy) due to feeding difficulty/inco-ordinate swallowing in children with cerebral palsy (n=214; 53.2%), and poor weight gain/failure to thrive (n=145; 36.1%), followed closely by those with chronic abdominal pain (n=103; 25.6%) and upper gastrointestinal bleeding (n=81; 20.1%). In patients who had combined gastroscopy with colonoscopy, the most common presenting symptoms were chronic abdominal pain (n=37; 47.5%), chronic bloody loose stools (n=35; 44.9%) in older children and chronic diarrhoea (n=30; 38.5%), as shown in Fig. 2.
In patients who underwent colonoscopy only, the most common presenting symptoms were rectal bleeding (n=13; 52.0%) and chronic bloody loose stools (n=9; 36.0 %) (Fig. 3).
Indications for endoscopy
Oesophagogastroduodenoscopy (OGD)
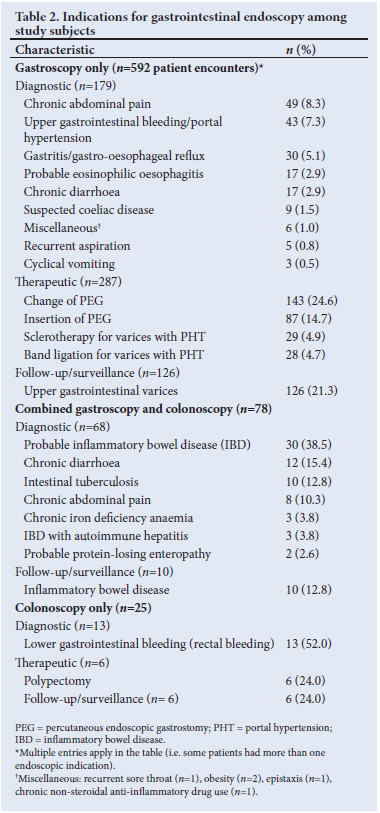
Among 592 gastroscopies performed, 179 (30.2%) were diagnostic, 287 (48.5%) therapeutic and 126 (21.3%) for follow-up/ surveillance.
The main diagnostic indications for gastroscopy were chronic abdominal pain (n=49; 8.3%), upper gastrointestinal bleeding/ portal hypertension with varices (n=43; 7.3%) and gastritis/gastro-oesophageal reflux (n=30; 5.1%), while the therapeutic indications for gastroscopy included insertion of PEG tube (n=87; 14.7%), change of PEG to gastrostomy tubes (n=143; 24.6%), variceal sclerotherapy of oesophageal varices (n=29; 4.9%) and variceal band ligation (n=28; 4.7%).
The follow-up/surveillance indications for OGD were mainly for previous upper gastrointestinal bleeding secondary to oesophageal varices, 204 (50.7%) (Table 2).
Combined OGD with colonoscopy
Of the 78 combined gastroscopy and colonoscopy procedures, the majority (n=68; 87.2%) were for diagnostic indications, which included probable inflammatory bowel disease (IBD; n=30; 38.5%), chronic diarrhoea (n=12; 15.4%), suspected intestinal tuberculosis (TB; n=10; 12.8%), chronic abdominal pain (n=8; 10.3%), chronic iron deficiency anaemia of unknown aetiology (n=3; 3.8%) and IBD screening in those with autoimmune hepatitis (n=3; 3.8%). The follow-up/surveillance indications were for inflammatory bowel disease (n=10; 12.8%) (Table 2).
Colonoscopy
Of 25 (100%) colonoscopy-only procedures undertaken, 13 (52%), 6 (24%) and 6 (24%) were for diagnostic, therapeutic and follow-up/surveillance indications, respectively.
Six (24%) therapeutic colonoscopy procedures were performed for polypectomy, while in another 6 (24%) cases, colonoscopies were undertaken for IBD follow-up/surveillance cases (Table 2).
Terminal ileum intubation and caecal examination rate
Terminal ileal intubation was attempted in diagnostic colonoscopy procedures undertaken as combined gastroscopy and colonoscopy or colonoscopy-only procedures as follows: 68 (87.2%) diagnostic combined gastroscopy with colonoscopy, 13 (52%) diagnostic and 6 (24.0%) follow-up/ surveillance colonoscopy-only procedures, totalling 87 patient procedures.
Of the 87 diagnostic colonoscopies performed, complete terminal ileum intubation with caecal examination was achieved in 85 (97.7%) cases. All cases of suspected IBD and/or small-bowel disease also had magnetic resonance enterography in addition to endoscopy.
Diagnostic yields
Gastroscopy
A total of 179 gastroscopies were for diagnostic purposes, of which 43 were for upper gastrointestinal bleeding, with the majority being oesophageal varices diagnosed macroscopically during endoscopy. Histology was only done in 5 of these cases. In all other cases, biopsies were taken and the histological results showed normal findings in 107 (18.1%), chronic gastritis in 33 (5.6%) and eosinophilic oesophagitis (EoE) in 7 (1.5%) (Fig. 4).
Colonoscopy
A total of 103 colonoscopies were undertaken, comprising 78 cases of combined gastroscopy with colonoscopy (68 being for diagnostic and 10 cases for follow-up surveillance) and 25 colonoscopies only (13 diagnostic, 6 therapeutic and 6 surveillance).
Initial diagnoses were considered. Of the 81 diagnostic colonoscopies (68 diagnostic combined gastroscopy with colonoscopy, and 13 colonoscopy only), multiple tissue biopsies were taken for histology (n=25; 24.3%) and had normal histological findings, and IBD was found in 19 (18.4%) cases, of which 10 (9.7%) were Crohn's disease and 9 (8.7%) ulcerative colitis.
Abnormal histopathological results were seen in biopsies taken from macroscopically normal-looking gastrointestinal mucosa (n=15), for diagnostic gastroscopies (n=15/179; 8.4%), combined gastroscopy and colonoscopy (n=6/68; 8.8%) and colonoscopies only (n=2; 15.4%) of the diagnostic procedures.
However, there was no statistically significant relationship between positive histological findings from tissue biopsies taken from macroscopically normal and abnormal gastrointestinal mucosae during gastroscopy (x2=6.419; p=0.526) and combined gastroscopies and colonoscopies (x2=5.142; p=0.275), respectively (Table 3).
Eight (9.9%) out of 10 probable cases of intestinal TB were diagnosed on histology. Chest radiographs showed evidence of healed TB (fibrosis and/or calcification) in 6 patients, and active pulmonary TB (presence of acid-fast bacilli in induced sputum) in 2 patients. Ulcerated areas and nodular friable mucosa were the most common lesions on colonoscopy. Mycobacterium tuberculosis was only cultured from three of the biopsies.
Histology
The majority (n=107; 18.1%) of tissue biopsies taken during gastroscopies had normal histological findings, although 33 (5.6%) showed chronic gastritis, 9 (1.5%) EoE and 7 (1.2%) Helicobacter pylori-associated gastritis (Fig. 4).
Normal histological findings were found in 25 (30.9%) diagnostic colonoscopies. IBD was diagnosed in 19 (23.5%) patients, made up of Crohn's disease (n=10; 12.3%) and ulcerative colitis (n=9; 11.1%), intestinal polyps in 9 (11.1%) and intestinal TB in 8 (9.9%) (Fig. 5).
Impact of gastrointestinal endoscopy on management
The various endoscopic procedures showed differing impacts on the management of cases in various ways. Out of 592 gastroscopies, 87 (14.7%) were done in patients with cerebral palsy or other neurological disorders with failure to thrive, and required PEG insertion for optimal feeding, while 57 (9.6%) had endoscopic treatment for oesophageal varices, which included sclerotherapy (n=29; 4.9%) and variceal band ligation (n=28; 4.7%). In addition, 8 (1.4%) and 2 (0.3%), respectively, had addition of new medication(s] and therapy for eradication of H. pylori following histological diagnoses. The majority (n=295; 49.8%) of cases had no significant findings on histology and did not require further treatment, and ongoing treatments were discontinued.
In 78 patients who underwent combined gastroscopy and colonoscopy procedures, 35 (44.9%) had addition of new medication(s) to their treatment, 5 (6.4%) were prescribed nutritional therapy using exclusive enteral nutrition for Crohn's disease/minimal fat diet, 2 (2.6%) change of medication(s) for differing gastrointestinal conditions, and 36 (46.1%) had no change or further treatment post colonoscopy based on review of histology and other results.
In 25 colonoscopies, 8 cases (32%) had change of medication(s) for different gastrointestinal conditions, and 5 (20%) addition of new medications. Six cases (24%) had polypectomy, and another 6 (24%) no change or no further treatment post colonoscopy, with review of histology and other results.
PEG insertion/change
Of the total of 230 PEG procedures performed during the period under review, 87 (37.8%) were PEG insertions, while 143 (62.2%) had change of the initial PEG to gastrostomy tubes. The indications for PEG insertion were feeding difficulty/inco-ordinate swallowing (mainly in children with neurological deficits, particularly cerebral palsy and traumatic brain injury) with failure to thrive/poor weight gain (n=85; 21.1%) and inco-ordinate swallowing at risk of poor medication (antiretroviral) adherence, 2 (0.5%) in patients with AIDS. PEGs were changed to a gastrostomy tube after a mean period of 3.7 (range 3 - 12) months. In 2 (1.4%) patients aged 7 and 10 years, respectively, initial PEG tubes were later changed to a MIC-KEY type of gastrostomy tube on request of the attending caregivers. There was significant increase in patients' weight upon their feeding using gastrostomy tubes post insertion. Among the 87 (37.8%) participants who had PEG insertion, the pre-PEG insertion mean weight was 11.4 kg, and increased to 13.5 kg at the time of change of PEG to gastrostomy tube (p<0.001).
Safety/complications
Endoscopic procedures undertaken among study participants were safe. Of a total of 695 endoscopies (592 gastroscopies, 78 combined gastroscopies with colonoscopies and 25 colonoscopies alone), complications occurred in 7 (1.0%). Most of these complications were relatedto the cardiovascular/respiratory system and anaesthetic.
Complications occurred post gastroscopy: pneumo-peritoneum in 1 patient (0.2%) post PEG insertion, desaturation in 1 (0.2%) and 1 (0.2%) failed extubation.
Amongthosewho underwentcombinedgastroscopy with colonoscopy procedures, out of 78 procedures, there were 3 complications: 1 child (1.3%) had stridulous breathing on extubation and another bradycardia/ hypotension, while in participants who had colonoscopy only (n=25), 1 case (4%) of bradycardia/hypotension was observed
Discussion
Recent advances in endoscopy designs and devices have made endoscopy an invaluable tool in diagnosis, therapy and follow-up/ surveillance of most gastrointestinal disorders in paediatric and child health practices.[2,13] Literature is scarce on paediatric gastrointestinal endoscopy in sub-Saharan Africa. It is hoped that experience gained in the present study will guide practice in many centres in the region and other parts of the developing world in setting up a paediatric gastrointestinal endoscopic service.
The present study is a comprehensive clinical audit of paediatric gastrointestinal endoscopy service undertaken by paediatric gastroenterologists and trainees in a paediatric specialist centre in Cape Town, SA.
Most patients in the current study were young, with only 2% (8) of them aged > 13 years. The hospital's cut-off for seeing new patients is age 13 years, and special permission must be sought to treat or continue to care for children >13 years old in the centre.
Most gastrointestinal disorders present with nonspecific signs and symptoms, making definitive diagnoses difficult without endoscopy in some cases.[8,13] However, there is a need to apply local experience and standard expert societal guidelines in centres running paediatric endoscopic services so as to improve diagnostic yields, which will ultimately impact on patient management. Abdominal pain was the most common indication for endoscopy among participants in the present study, which has been corroborated by authors in most similar studies.[16,19] Also, evaluation for PEG insertion in children with feeding difficulties/inco-ordinate swallowing and poor weight gain/failure to thrive were the prevalent therapeutic indications for gastroscopy. PEG tubes were mainly inserted for improved feeding in cases of inco-ordinate swallowing due to neurological disorders. Sclerotherapy or band ligation for oesophageal varices were the second-most common indications for therapeutic gastroscopy in the current study. Rectal bleeding and chronic bloody loose stools were the two leading indications for colonoscopy in the present study, and this has also been corroborated by other studies.[21,22] The experience in the current study also corroborates the recommendations by key expert societal guidelines on common indications for performing endoscopy in children,[13] based on the presenting symptoms, and underscores the need to adhere to them for utmost endoscopic impacts and outcomes. Combined gastroscopy and colonoscopy procedures are the benchmark for the diagnosis and follow-up of some paediatric gastrointestinal disorders, particularly IBD (Crohn's disease, ulcerative colitis or indeterminate IBD), polyposis and eosinophilic colitis, among others,[23,24] as found in the present study Endoscopy is an important diagnostic and therapeutic tool in children. Various gastrointestinal disorders have been diagnosed with the aid of endoscopy, as in the current study. Some diagnoses were made macroscopically during endoscopy, e.g. oesophageal and gastric varices, EoE with concentric ring formation/trachealisation and longitudinal linear furrows and patches of small, white papules on the oesophageal surface (confirmed on histology),[25] while others were diagnosed from histopathology on biopsy specimens taken during endoscopy. Some therapeutic endoscopy procedures were employed, including sclerotherapy (in the younger infants/toddlers) and variceal band ligation (in older children), in managing cases of upper gastrointestinal bleeding from oesophageal varices and polypectomy for juvenile polyps.
In addition, follow-up scopes were done in cases of IBD to assess disease remission and relapses. Follow-up surveillance was done in cases of multiple juvenile polyposis for monitoring of development of colorectal carcinoma, as there is a 15% incidence of such malignancy in patients <35 years of age.[22,23]
Overall endoscopic yield for the various modalities of upper and lower endoscopy in the present study was high. The high endoscopic yield observed may be due to the appropriate selection of cases with correct indications for endoscopy using standard societal guidelines,[26] pre-procedure preparations including standard bowel preparations, as well as obtaining of biopsies at the time of endoscopy from both macroscopically normal- and abnormal-looking gastrointestinal mucosa. High diagnostic yields have equally been reported for OGD and colonoscopy in similar studies. [18,19,27,28]
Using standard societal guidelines, including the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) and North American Society for Paediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN), the correct indications for endoscopy will lead to a high impact rate on management of children with various gastrointestinal symptoms/ disorders. Significant impacts on patient management were seen in patients with PEG insertions for feeding difficulties.
Improved oral intake allowed these children to meet their recommended dietary allowances, with attendant improved growth as evidenced by an increase in median weight-for-age z-scores (-2 - 0). Similar findings have been reported in other studies.[29,30] Children with portal hypertension and oesophageal varices causing upper gastrointestinal bleeding also benefited from either sclerotherapy or variceal band ligation, with good results depending on their age.[31]
In addition, a few of the patients in the current study with juvenile polyposis had snare polypectomy and subsequent follow-up/ surveillance for possible development of associated malignancies.
There were cases in the present study where changes in treatment were made based on endoscopy. In cases of IBD with relapsing or worsening disease activity based on either paediatric ulcerative colitis activity index scores[32] or Crohn's disease activity index scores,[33] escalation of medical therapy was required to improve the clinical outcome.
In a study by Thakkar et al.,[9] a 42% change in patients' management was made in their study, made up of a 20% change in management immediately following endoscopy and 18% post histology review and 9% after both, respectively. In their study, management changes were mainly the addition of new medication(s) in children with IBD, particularly those with Crohn's disease, with improvement in their overall treatment outcomes, similar to the experience in the present study. Other benefits of endoscopies in our study participants included eradication of H. pylori in cases diagnosed on histology, and culture of the gastric antrum biopsies, as well as polypectomies of juvenile polyps, among others. Most of these gastrointestinal endoscopic impacts on management have been corroborated by researchers in similar studies.[25,29 31,34]
In paediatric endoscopies, it is advised to take biopsy specimens from both macroscopically normal- and abnormal-looking gastrointestinal mucosae for histology. This is because some gastrointestinal mucosa may appear macroscopically normal on endoscopy, but show pathology/abnormal histology. It has been reported that in -20% of macroscopically normal upper gastrointestinal mucosa, biopsies reveal various pathological conditions on histology, thereby improving the rate of endoscopic yields in such cases.[35]
Limitations exist in gastroscopy and colonoscopy (diagnostic) in the evaluation of various gastrointestinal complaints in children, as some studies have reported no histological abnormalities in up to 60% of the sites biopsied, and in -65%, no macroscopic abnormalities observed during endoscopy.[36] Negative histopathological findings on biopsies are useful in excluding pathology and thus reassuring and relieving anxiety in patients and their families,[28] as well as averting the need for further investigations, with resulting lowered economic costs in patient management. Considering the potential complications and costs of gastrointestinal endoscopy under general anaesthesia as in the current centre, appropriate clinical judgement and guidelines should be applied in selecting patients with the right indications for endoscopy.[37]
Terminal ileum intubation is an important indicator of complete colonoscopy. It is invaluable in the diagnosis of some specific gastrointestinal disorders, including IBD, intestinal TB and chronic diarrhoea, among others, that affect the gut.[38,39]
The terminal ileum intubation rate of 97.7% observed in the current study appears to be much higher than findings in similar studies,[38,39] and could have played a significant role in the overall outcome of the study. It is possible that the high level of bowel preparations in patients who underwent colonoscopy as well as the endoscopic skills of the experienced gastroenterologists resulted in the high rate of terminal ileum intubation. The histopathology of terminal ileal biopsies was essential in distinguishing gastrointestinal disorders, including distinguishing intestinal TB from Crohn's disease, in which the former occurs commonly in the ileo-caecal gut.
Most cases of intestinal TB (n= 8; 9.9%) seen in the present study had histological features of the disease. TB is endemic in SA and is treatable, with good outcomes using anti-tuberculous agents for 6 months.[40] Patients with Crohn's disease may have coexisting latent TB, which is important to exclude as IBD immunosuppressive treatment could result in reactivation of latent TB and further disseminated TB. Intestinal TB needs to be excluded in our setting before starting immunosuppressive treatment in IBD cases.
There was a low complication rate recorded in the present study, with no mortality attributable to the endoscopic procedures reported. RCWMCH has an experienced anaesthetic department, and all paediatric gastrointestinal endoscopies were performed under general anaesthesia. Though a few cases of anaesthetic-related minor complications were reported, no mortality was recorded compared with use of intravenous sedation for endoscopy
The majority of the complications (n=5/7; 71.4%) reported in the current study were anaesthetic related, as has been observed by other studies.[30,42] It is plausible that the use of standard expert societal guidelines in selecting patients for endoscopy in the current study improved pre-endoscopic preparations of patients, and that performance of the procedures by a gastroenterologist and/or trainee fellows under general anaesthesia administered by a consultant anaesthetist accounted for the low complication rate observed.
Conclusion
Endoscopy offers diagnostic and therapeutic options in children. Positive histological reports were recorded in some cases where gastrointestinal mucosae appeared normal. There is a need to obtain biopsies from both abnormal-looking and macroscopically normal mucosa, as significant histology could be made from these, hence improving diagnostic yield.
One limitation of the present study is that the retrospective nature of a longitudinal study might have revealed long-term outcomes of some of the cases.
Declaration. This study was required for the purposes of an M Phil degree (Paediatric Gastroenterology) in the Faculty of Health Sciences, University of Cape Town, South Africa.
Acknowledgements. CBE: my sincere thanks to Almighty God for his grace throughout my training period at the University of Cape Town.
Author contributions. CBE: conceptualisation of the study, literature review/proposal writing, acquisition of data, data analysis, initial manuscript draft and critical editing of the manuscript for important intellectual content. EAG: conceptualisation of the study, supervision and critical editing of the manuscript for important intellectual contents. RAB and RJD: supervision and critical editing of the manuscript for important intellectual content.
Funding. None.
Conflicts of interest. None.
References
1. Tringali A, Thomson N, Dumonceau JM, et al. Pediatric gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) and European Society for Paediatric Gastroenterology Hepatology and Nutrition Guideline Executive Summary. Endoscopy 2017;49(1):83-91. https://doi.org/10.1055/S-0042-111002 [ Links ]
2. Wang S, Younas O, Rawat D, et al. Clinical presentation and outcomes of diagnostic endoscopy in newly presenting children with gastrointestinal symptoms. J Pediatr Gastroenterol Nutr 2018; 66(6):876-81. https://doi.org/10.1097/mpg.0000000000001864 [ Links ]
3. Nel ED. A brief review of the history of paediatric gastroenterology. S Afr Gastroenterol Rev 2013;11(2):16-18. [ Links ]
4. Belsha D, Bremner R, Thomson M. Indications for gastrointestinal endoscopy in childhood. Arch Dis Child 2016;101(12):1-8. https://doi.org/10.1136/archdischild-2014-306043. [ Links ]
5. FriedtM, Welsch S. An update on paediatric endoscopy. Eur J Med Res 2013; 18(1) 24. https://doi.org/10.1186/2047-783x-18-24 [ Links ]
6. Rubio-Tapai A, Hill ID, Kelly CP, Calderwood AH, Murray JA. ACG Clinical guidelines: Diagnosis and management of celiac disease. Am J Gastroenterol 2013;108(5): 656-676. https://doi.org/10.1038/ajg.2013.79 [ Links ]
7. Eke CB. Advances in gastrointestinal endoscopy: Shaping diagnosis and therapy of gastrointestinal disorders in children. Niger J Pediatr 2019;46(3):124-128.http://doi.org/10.4314/njp.v46i3.1 [ Links ]
8. American Society for Gastrointestinal Endoscopy (ASGE) Standards of Practice Committee, Lightdale JR, Acosta R, et al. American Society for Gastrointestinal Endoscopy. Modifications in endoscopic practice for pediatric patients. Gastrointest Endosc 2014;79(5);699-710. https://doi.org/10.1016/j.gie.2013.08.014 [ Links ]
9. Thakkar K, Lucia CJ, Ferry GD, McDuffie A, Watson KL. Repeat endoscopy affects patients management in paediatric inflammatory bowel disease. Am J Gastroenterol 2009;104(3):722-727. https://doi.org/10.1038/ajg.2008.111 [ Links ]
10. Chang MT, Wang TH, Jsu JY, Lee TC, Wang CY, Yu JY. Endoscopic examination of the upper gastro-intestinal tract in infancy. Endosc 1983;29(1):15-17. [ Links ]
11. Suzuki H, Kato J, Kuriyama M, Hiraoka S, Kuwaki K, Yamamoto K. Specific endoscopic features of ulcerative colitis complicated by cytomegalovirus infection colitis. World J Gastroenterol 2010;16(10):1245-1251. https://doi.org/10.3748/wjg.v16.i10.1245 [ Links ]
12. Cox CB, Laborda T, Kynes JM, Hiremath G. Evolution in the practice of pediatric endoscopy and sedation. Front Pediatr2021;14(9):687635. https://doi.org/10.3389/fped.2021.687635 [ Links ]
13. Thomson M, Tringali A, Dumonceau J-M, et al. Paediatric gastrointestinal endoscopy: European Society of Paediatric Gastroenterology Hepatology and Nutrition (ESPGHAN) and European Society of Gastrointestinal Endoscopy Guidelines (ESGE). J Pediatr Gastroenteerol Nutr 2017;64(1):133-153. https://doi.org/10.1097/mpg.0000000000001408 [ Links ]
14. Guariso G, Meneghel A, Pozza LVD, et al. Indications for gastrointestinal endoscopy in children with dyspepsia. J Pediatr Gastroenterol Nutr 2010;50(5):493-499. https://doi.org/10.1097/mpg.ObO13e3181bb3362 [ Links ]
15. Pall H, Lerner D, Khlevner J, et al. Developing the pediatric gastrointestinal endoscopy unit: A clinical report by the endoscopy and procedures committee. J Pediatr Gastroenterol Nutr 2016:63(2):295-306. https://doi.org/10.1097/mpg.0000000000001189 [ Links ]
16. Kelly P, Katema M, Amadi B, et al. Gastrointestinal pathology in the University Teaching Hospital, Lusaka, Zambia: Review of endoscopic and pathology records. Trans Royal Soc Trop Med Hyg 2008;102(2):194-199. https://doi.Org/10.1016/j.trstmh.2007.10.006 [ Links ]
17. Adeniyi OF, Lesi OA, Odeghe FA, Adekola O, Oduwole O. Upper gastrointestinal endoscopy in children: The Lagos University Teaching Hospital experience. S Afr J Child Health 2016;10(4):207-201. https://doi.org/10.7196/SAJCH.2016.v10i4.1116 [ Links ]
18. Mudawi HM, El Tahir MA, Suleiman SH, et al. Pediatric gastrointestinal endoscopy: Experience in a Sudanese university hospital. East Mediterr Health J 2009;15 (4):1027-1031. https://doi.Org/10.26719/2009.15.4.1027 [ Links ]
19. Lyons H, Zhang Y, Szpunar S, Dhamaraj R. Predictors of positive esophagogastroduodenoscopy outcomes in children and adolescents: A single centre experience. BMC Res Notes 2017;10(1):356. https://doi.org/10.1186/S13104-2693-7 [ Links ]
20. Western Cape Department of Health. Western Cape Government Provincial Gazette Extraordinary 77752. Uniform patient fee schedule regulation for health services rendered by the Western Cape Department of Health. 31 March 2017. [ Links ]
21. Vazeou A, Papadopoulou A, Booth IW, Bartsocas CS. Prevalence of gastrointestinal symptoms in children and adolescents with type 1 diabetes. Diabetes Care 2001;24(5):962-964. https://doi.Org/10.2337/diacare.24.5.962 [ Links ]
22. Lei P, Gu F, Hong L, et al. Pediatric colonoscopy in South China: A 12-year experience in a tertiary centre. PLoS One 2014;9(4):e95933. https://doi.org/10.1371/journal.pone.0095933 [ Links ]
23. Yoshioka S, Takedathus H, Fukunaga S, et al. Study to determine guidelines for pediatric colonoscopy. World J Gastroenterol 2017;23(31):5773-5779. https://doi.org/10.3748%2Fwjg.v23.i31.5773 [ Links ]
24. Lee WS, Zainuddin H, Boey CCM, Chai PF. Appropriateness, endoscopic findings and contributive yield of pediatric gastrointestinal endoscopy. World J Gastroenterol 2013;19(47):9077-9083. https://doi.org/10.3748%2Fwjg.vl9.i47.9077 [ Links ]
25. Crowley E, Hussey S. Helicobacter pylori in childhood. In: Wyllie R, Hyams JS, Kay M (editors). Pediatric Gastrointestinal and Liver Disease. 5th edition. Philadelphia: Elsevier, 2016:309-327. [ Links ]
26. Thomson M, Sharma S. Diagnostic yield of upper and lower gastrointestinal endoscopies in children in a tertiary centre. J Pediatr Gastroenterol Nutr 2017;64(6):903-908. https://doi.org/10.1097/mpg.0000000000001582 [ Links ]
27. Thakker K, Holub JL, Gilger MA, et al. Quality indicators for pediatric colonoscopy results from a multi-center consortium. Gastrointest Endosc 2016;83(3):533-541. https://doi.Org/10.1016/j.gie.2015.06.028 [ Links ]
28. Wu CT, Chen CA, Yang YJ. Characteristics and diagnostic yield of pediatric colonoscopy in Taiwan. Pediatr Neonatol 2015;56(5):234-238. https://doi.org/10.1016/j.pedneo.2015.01.005 [ Links ]
29. Wu FY, Wu JF, Ni YH. Long-term outcome after percutaneous gastrostomy in children. Pediatr Neonatol 2013;54(5):326-329. https://doi.org/10.1016/j.pedneo.2013.04.008 [ Links ]
30. Sharma R, Williams AN, Zaw W. Timing of gastrostomy insertion in children with a neuro-disability: A cross-sectional study of early versus late intervention. BMJOpen 2012;2:e001793. https://doi.org/10.1136/bmjopen-2012-001793 [ Links ]
31. Itha S, Yachha SK. Endoscopic outcome beyond esophageal variceal eradication in children with extrahepatic portal vein obstruction. J Pediatr Gastroenterol Nutr 2006;42(2):196-200. https://doi.org/10.1097/01.mpg.0000189351.55666.45 [ Links ]
32. Kerur B, Litman HJ, Stern JB, et al. Correlation of endoscopic disease severity with pediatric ulcerative colitis activity index score in children and young adults with ulcerative colitis. World J Gastroenterol 2017;23(18):3322-3329. https://doi.org/10.3748/wjg.v23.il8.3322 [ Links ]
33. Hyams J, Markowitz J, Otley A, et al. Evaluation of the pediatric Crohn Disease Activity Index: A prospective multicenter experience. J Pediatr Gastroenterol 2005;41(4):416-421. https://doi.org/10.1097/01.mpg.0000183350.46795.42 [ Links ]
34. Lin CH, Wu RSC, Lin WC, Wu SF, Chen AC. Colonoscopic polypectomy of colorectal polyps in children under general anaesthesia. Kovohsiung J Med Sci 2009;25(2):70-76. https://doi.org/10.1016/s1607-551x(09)70043-9 [ Links ]
35. Eke CB, Brown RA, De Lacy RJ, Pillay K, Goddard EA. Collagenous gastritis: An unusual cause of generalised oedema in a child. J Tropical Pediatrics 2019;65(3):305-308. https://doi.org/10.1093/tropej/fmy048 [ Links ]
36. Sheiko MA, Feinstein JA, Capocelli KE, Kramer RE. Diagnostic yield of oesophagogastroduodenoscopy in children: A retrospective single centre study of 10 000 cases. Gastrointest Endosc 2013:78(1):47-54. https://doi.org/10.1016%2Fj.gie.2013.03.168 [ Links ]
37. O'Loughlin EV, Dutt S, Kamath R, Gaskin K, Domey S. Prospective peer-review audit of paediatric upper gastrointestinal endoscopy. J Paediatr Child Health 2007:43(7-8):551-554. https://doi.Org/10.1111/j.1440-1754.2007.01132.x [ Links ]
38. Oliva S, Thomson M, de Ridder L, et al. Endoscopy in pediatric IBD: A position paper on behalf of the Porto IBD Group of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;67(3):414-430. https://doi.org/10.1097/mpg.0000000000002092 [ Links ]
39. Lee WS, Tee CW, Koay ZL, et al. Quality indicators in pediatric colonoscopy in a low-volume center: Implications for training. World J Gastroenterol 2018;24(9):1013-1021. https://doi.org/10.3748/wjg.v24.19.1013 [ Links ]
40. Keane J,Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, tumour necrosis factor alpha-neutralizing agent. N Engl J Med 2001;345(15):1098-1104. https://doi.org/10.1056/nejmoa011110 [ Links ]
41. Abdool Karim SS, Churchyard GJ, Abdool Karim Q, Lawn SD. HIV infection and tuberculosis in South Africa: An urgent need to escalation the public health response. Lancet 2009;374(9693):921-933. https://doi.org/10.1016%2FS0140-6736(09)60916-8 [ Links ]
42. Ammar MS, Pfefferkorn MD, Gome JM, Gupta SK, Corkins MR, Fitzgerald JF. Complications after outpatient upper gastrointestinal endoscopy in children: 30-day follow-up. Am J Gastroenterol 2003;98(7):1508-1511. https://doi.org/10.1111/j.1572-0241.2003.07524.x [ Links ]
 Correspondence:
Correspondence:
C B Eke
christopher.eke@unn.edu.ng
Accepted 31 May 2021














