Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.16 no.1 Pretoria Abr. 2022
http://dx.doi.org/10.7196/sajch.2022.v16i1.1800
SHORT REPORT
Trends in neonatal mortality in a regional hospital in the Eastern Cape, South Africa: Quality improvement in action
C A MackayI; S MdaI; F KhanII; S MaharajIII; J S SmitIV; N MakhubaloV; N JezileVI
IPhD; Dora Nginza Hospital, Gqeberha, South Africa
IIFCPaed; Dora Nginza Hospital, Gqeberha, South Africa
IIIFCPaed, Cert Neonatol; Dora Nginza Hospital, Gqeberha, South Africa
IVMMed; Dora Nginza Hospital, Gqeberha, South Africa
VMSc Health Sciences; Nelson Mandela Bay Municipality, Gqeberha, South Africa
VIDip Neonatal Nursing Science, Dip Child Nursing Science; Dora Nginza Hospital, Gqeberha, South Africa
ABSTRACT
BACKGROUND: Dora Nginza Hospital had a high neonatal mortality rate (NMR) in 2016. Quality improvement (QI) strategies were subsequently introduced to improve outcomes
OBJECTIVE: To report changes in the NMR at Dora Nginza Hospital from 2016 to 2019, following the introduction of QI interventions
METHODS: A retrospective comparison was conducted of unit-based data from before and after the introduction of QI interventions. Outcomes included total, early and late NMR, NMR by birthweight categories and causes of neonatal deaths. A chi-squared test and relative risk were used to compare groups, with p<0.05 considered significantly different
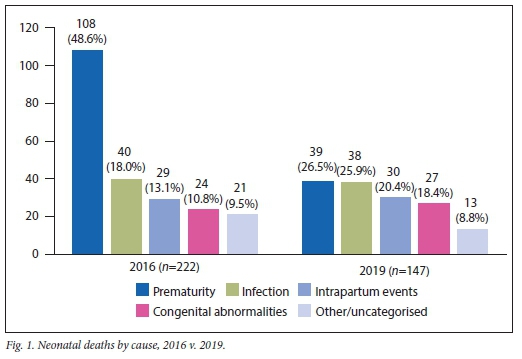
RESULTS: Total NMR declined from 34.4/1 000 live births to 19.4/1 000 (p<0.01). Early NMR decreased across all birthweight categories (p<0.01). Late NMR decreased in neonates >500 g (p=0.02) but not in those >1 000 g (p=0.99). Relative risk of early or late neonatal death was 0.57 (0.46 - 0.69). Total deaths due to prematurity decreased from 108 in 2016 to 39 in 2019 (p<0.01). There was no significant change in deaths due to congenital abnormalities (p=0.051), infection (p=0.1) or intrapartum events (p=0.08
CONCLUSION: We report a significant reduction in NMR following QI interventions, largely due to a decrease in prematurity-related early neonatal mortality. Similar interventions may be beneficial in other poorly resourced settings. Adequate kangaroo-mother care facilities, availability of nasal continuous positive-pressure ventilation to all preterm neonates, promotion of breastfeeding and protocol-driven management of premature newborns are key
Neonatal mortality remains high despite international efforts to reduce it. The average global neonatal mortality rate (NMR) was 17/1 000 live births in 2019 but discrepancies exist between highland low-income countries.1 NMR ranges from 1/1 000 in certain high-income countries to 52/1 000 in some low-income countries.1Countries with the highest NMRs need support to meet the global goal of 12/1 000 live births.2
South Africa (SA) is classified as an upper middle-income country and has an average NMR of 11/1 000 live births.1,3However, inequalities exist in SA, with differences both between the country's provinces and between districts within provinces. 3,4 Across provinces, the rate for early neonatal mortalities ranges from 6.9/1 000 in the Western Cape to 14.2/1 000 in the Northern Cape. Across districts in the Eastern Cape, the rate for early neonatal mortalities ranges from 7.5/1 000 to 17/1 000.3
Birthweight and postnatal age further impact survival. Infants weighing <1000 g at birth and those <7 days old are at highest risk.3 The NMR for babies weighing 500 - 999 g in SA is 547.9/1 000 live births compared with 3.2/1 000 in those who weigh >2 500 g.3 Furthermore, early neonatal deaths (in the first seven days of life) account for -80% of all neonatal deaths.3
Dora Nginza Hospital (DNH) in the Nelson Mandela Bay district Eastern Cape Province has historically had one of the highest NMRs in SA, with 34.4 neonatal deaths per 1 000 live births being recorded in 2016. A quality-improvement (QI) process was subsequently instituted to reduce neonatal mortality.
The aim of this study was to report changes in neonatal mortality at DNH from 1 January 2016 to 31 December 2019, following the introduction of several QI strategies.
Methods
Study design
A retrospective review of monthly unit-based neonatal statistics and morbidity and mortality audits was conducted.
Study population
Records of neonates <28 days of life and who had been admitted to the neonatal unit at DNH between 1 January 2016 and 31 December 2019 were included in the review. Records from those admitted to the general paediatric wards were excluded.
Study setting
The neonatal unit at DNH has 72 beds. In 2016, six were intensive care beds and 10 were allocated for kangaroo-mother care (KMC); the remainder were all standard-care beds. The facility did not have a neonatal high-care unit. There were ~24 000 deliveries in the catchment area in 2016, of which 6 457 were at DNH. The unit handled a total of 5 317 admissions, both from DNH and the broader catchment area, in the same period. The majority of the patients, with rare exceptions, were fed breast milk (either their mother's own or milk donated from an on-site milk bank). The unit was overcrowded and patient care was poorly organised: different parts of the unit were located in different areas of the hospital and well lodger' babies (babies unable to stay with their mothers owing to maternal illness), standard-care patients and high-care patients were all cared for together.
Quality-improvement process
Problem areas identified before instituting the QI process included: issues of overcrowding; poor organisation of care; inadequate use of district hospitals; lack of treatment protocols; and high infection rates. The following interventions were subsequently implemented and progress was reviewed at weekly meetings, with adjustments made as necessary.
1. Reorganisation of the neonatal unit
• Well babies of 2 000 - 2 499 g, previously routinely admitted to the neonatal unit, were admitted with their mothers and monitored in the postnatal ward.
• Layout and allocation of beds were reorganised according to level of care (the total number of beds was unchanged):
• Beds for KMC were increased from 10 to 18 and facilities were optimised.
• A high-care area with 16 beds was set up.
• A triage area of eight beds was established for short-stay admissions (up to 24 hours).
2. Upskilling at district hospitals
• Nasal continuous positive airway pressure (CPAP) was implemented in district hospitals.
• Outreach was commenced.
• Staff training included the 'Helping babies breathe' and 'managing small and sick newborns' modules of the American Academy of Paediatrics' educational programme.
3. Clinical protocols
• Management protocols were developed.
• Guidelines for the care of extremely premature neonates included fluid and electrolyte management, maintaining normothermia, provision of CPAP to all neonates >500 g, selective surfactant treatment in patients <800 g and feeding guidelines. These guidelines were primarily implemented at DNH. Guidelines regarding maintenance of normothermia, fluid management, feeding and use of CPAP were shared with district hospitals during outreach or when consulting on individual patients.
4. Infection prevention and control (IPC)
• Healthcare workers were trained on IPC measures.
• Evidence-based care bundles were introduced, including for central line-associated bloodstream infection and ventilator-associated pneumonia.
• An antimicrobial stewardship programme was implemented.
5. Morbidity and mortality audits
• Neonatal deaths were reviewed weekly according to the Perinatal Problem Identification Programme format to verify cause of death, identify modifiable factors and monitor the impact of QI interventions.
QI interventions were introduced in stages. Morbidity and mortality meetings started on 1 April 2017, with reorganisation of the unit, and outreach to district hospitals and development of protocols following from 1 June 2017. IPC interventions were introduced from 1 August 2018 after healthcare worker-associated infections were identified as a major cause of mortality.
Neonatal deaths were recorded using a template completed by the attending doctor. Cause of death and modifiable factors were verified by the neonatal team (which included at least one neonatologist, two paediatricians, four medical officers/registrars and four senior nursing staff). Interventions and issues arising were discussed, reviewed and recorded by the nursing manager for the area at the weekly meetings. Outcome measures assessed included early, late and total NMRs.
Data collection and analysis
Statistics for the neonatal unit, derived from ward registers and weekly mortality audit reports, were reviewed. Data on live births and stillbirths, verified at monthly data verification meetings, were obtained from the hospital's database. Data for 2016 were used as baseline and differences were compared using a chi-squared test and relative risk. A significance level of p<0.05 was used.
Ethical considerations
Approval for the study was granted by Walter Sisulu University (ref. no. 001/2020) and the Eastern Cape Department of Health (ref. no. EC_202002_003).
Results
As shown in Table 1, the total NMR declined from 34.4/1 000 live births in 2016 to 19.4/1 000 live births in 2019 (p<0.01). Over the same period, early NMR decreased by 46.7% and 44.4% in neonates >500 g and >1 000 g, respectively (p<0.01). Late NMR decreased by 36.3% in neonates >500 g (p=0.02) but no statistically significant difference was noted in those >1 000 g (p=0.99). Total NMR decreased by 43.6% (p<0.01) and 30.7% (p=0.01) in neonates > 500 g and >1 000 g, respectively.
There was a borderline decrease in the rate of stillbirths (17.4% (p=0.05)) and a significant decrease in perinatal mortality rate (29.4% (p<0.01)) for the period.
Deaths due to prematurity decreased from 108 to 39 (63.9%; p<0.01). There was no significant difference in deaths due to infections (p=0.10), intrapartum events (p=0.08) or congenital abnormalities (p=0.051).
Discussion
DNH has historically had one of the highest neonatal mortality figures in SA. The introduction of several low-cost, high-impact strategies resulted in significantly reduced neonatal mortality, mainly owing to a reduction in early neonatal deaths due to prematurity Stillbirths did not increase and perinatal mortality decreased, which indicate the reflected trend to be a true reduction in NMR.
The greatest reduction in mortality was seen in the early neonatal period, mainly owing to a decrease in prematurity-related deaths. This was likely because of improved care of very and extremely preterm infants following the introduction of detailed clinical protocols, availability of CPAP to all neonates >500 g, KMC facilities being expanded and the introduction of clinical audits. However the reduction in the early NMR, specifically due to prematurity, may also reflect improvements in antenatal care and increased uptake of antenatal steroids.5 Although no such interventions were introduced during the study period, these cannot be excluded as potential confounders.
Healthcare worker-associated infections were identified as a major cause of mortality in weekly audit meetings. IPC
training, care bundles and antimicrobial stewardship measures were subsequently introduced. Unfortunately this did not lead to a reduction in infection-related deaths. Several infection-prevention strategies have been proposed and deserve further attention. These include prevention of antibiotic overuse, implementation of evidence-based care bundles, rationalisation of invasive diagnostic and therapeutic procedures, reducing overcrowding and optimising staff-to-patient ratios.6
Mortality related to intrapartum events did not decrease. This agrees with findings from the SA 'Saving Babies 2014 - 2016' report, which shows no decrease in mortality due to intrapartum asphyxia.3 As the current QI project focused on postpartum neonatal interventions, we would not expect a decline in mortality due to intrapartum events. However, intrapartum asphyxia is a major preventable cause of morbidity, mortality and increasing litigation, and should be a key focus for future QI initiatives.3,7
The majority of neonatal patients at DNH are fed breastmilk (either their mothers' or donations) and promotion of breastfeeding is standard. This aspect was therefore not identified as part of the QI programme. However, the benefits of breastfeeding, ideally with mothers' own milk, should not be underestimated and the promotion of breastfeeding should continue to be prioritised.8
Study limitations
The introduction of QI strategies overlapped and the study was not designed to assess the impact of each intervention independently. Data from neonates admitted to general paediatric wards were excluded, which not only affected mortality calculations, especially late NMR, but also made it difficult to verify unit-based data against district and hospital databases. Data collection was retrospective and certain information was unavailable. Data on patients born at DNH compared with those transferred after delivery were not well documented and could not be analysed. The impact of district-level interventions on transfers and overcrowding therefore could not be assessed. It would also have been useful to have a breakdown of mortality by birthweight in the 500 - 1 000 g category, but this information was not available.
Conclusion
The study reports a significant reduction in NMR at a regional hospital in the Eastern Cape, largely due to decreased prematurity-related early neonatal mortality following several low-cost, high-impact interventions. Similar QI interventions may be beneficial in other poorly resourced settings. Adequate KMC, availability of nasal CPAP to all preterms, promotion of breastfeeding and protocol-driven management are key.
Declaration. None.
Acknowledgements. We thank the medical and nursing staff of the neonatal unit at DNH who implemented the QI interventions.
Author contributions. The study was conceptualised by CM, S Mda, FK and S Maharaj. These authors were also responsible for data collection and statistical analysis, assisted by IS, NM and NJ. All authors contributed to manuscript preparation.
Funding. None.
Conflicts of interest. None.
References
1. United Nations Inter-Agency Group for Child Mortality Estimation. Levels and trends in child mortality: Report 2020, Estimates developed by the United Nations Inter-Agency Group for Child Mortality Estimation. New York: UNICEF WHO, World Bank Group and United Nations, 2020. https://www.unicef.org/media/79371/file/UN-IGME-child-mortality-report-2020.pdf (accessed 22 January 2021). [ Links ]
2. United Nations. Transforming our world: The 2030 agenda for sustainable development. New York: United Nations, 2015. https://sustainabledevelopment.un.org/post2015/transformingourworld (accessed 20 September 2018). [ Links ]
3. National Perinatal Morbidity and Mortality Committee. Saving Babies 2014 - 2016: Triennial report on perinatal mortality in South Africa. South Africa: National Parinatal Morbidity and Mortality Committee, 2018. https://www.westerncape.gov.za/assets/departments/health/napemmco_triennial_report_2014-2016_saving_babies.pdf (accessed 22 January 2021). [ Links ]
4. World Bank Group. The world by income. https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html (accessed 29 January 2021). [ Links ]
5. Dixon CL, Too G, Saade GR, Gyamfi-Bannerman C. Past and present: A review of antenatal corticosteroids and recommendations for late preterm birth steroids. Am J Perinatal 2018;35(13):1241-1250. https://doi.org/10.1055/s-0038-1653944 [ Links ]
6. Wojkowska-Mach J, Chmielarczyk A, Strus M, Lauterbach R, Heczko P Neonate bloodstream infections in Organisation for Economic Cooperation and Development Countries: An update on epidemiology and prevention. J Clin Med 2019;8(10):1750. https://doi.org/10.3390/jcm8101750 [ Links ]
7. Coetzee M. Hypoxic-ischaemic encephalopathy: Identifying newborns who will benefit from therapeutic hypothermia in developing countries. S Afr J Child Health 2018;12(4):175-180. https://doi.org/10.7196/SAJCH.2018.vl2i4.1539 [ Links ]
8. Underwood MA. Human milk for the premature infant. Pediatr Clin North Am 2013;60(l):189-207. https://doi.org/10.1016/j.pcl.2012.09.008 [ Links ]
 Correspondence:
Correspondence:
S Mda
siyazi.mda@gmail.com
Accepted 30 March 2021














