Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Child Health
versión On-line ISSN 1999-7671
versión impresa ISSN 1994-3032
S. Afr. j. child health vol.16 no.1 Pretoria abr. 2022
http://dx.doi.org/10.7196/sajch.2022.v16.i1.1818
RESEARCH
Challenges in the provision of tuberculosis preventive therapy to children in Gauteng Province, South Africa
R T PhilipI; U D FeuchtII, III, IV, V
IMB ChB, MMed (Paeds); Department of Paediatrics and Child Health, University of Pretoria and Kalafong Hospital, Pretoria, South Africa
IIFCPaeds (SA), PhD; Department of Paediatrics and Child Health, University of Pretoria and Kalafong Hospital, Pretoria, South Africa
IIIFCPaeds (SA), PhD; Tshwane District Clinical Specialist Team (DCST), Gauteng Department of Health, City of Tshwane, South Africa
IVFCPaeds (SA), PhD; Research Centre for Maternal, Fetal, Newborn & Child Health Care Strategies, University of Pretoria, Pretoria, South Africa
VFCPaeds (SA), PhD; Maternal and Infant Health Care Strategies Unit, South African Medical Research Council, Pretoria, South Africa
ABSTRACT
BACKGROUND: Tuberculosis preventive therapy (TPT) offered to children who come into contact with infectious adult pulmonary tuberculosis (TB) cases is an important childhood TB prevention strategy
OBJECTIVES: To document paediatric TPT coverage as per South African national TB guidelines, to measure basic knowledge of TPT in adult TB patients and healthcare workers (HCWs), and to determine challenges in TPT delivery in eligible children
METHODS: We conducted a descriptive, cross-sectional study at primary healthcare clinics in South-West Tshwane, Gauteng Province, South Africa (SA). Structured interviews were conducted with adult TB patients to obtain socio-demographic data, TB and HIV history, data on child contacts and TPT knowledge. A separate questionnaire probed HCWs' knowledge of TPT. Patient folders and the clinical process flow of adult TB cases and children on TPT were also assessed
RESULTS: We interviewed 100 adult TB patients and identified 28 child contacts who were eligible for TPT, including six children (21%, n=6/28) on TPT, all HIV-uninfected and <5 years of age. Instability in household configuration was the most common reason for eligible children not having been brought to health facilities for assessment (57%; n=4/7). Almost all adult TB patients were aware of their TB diagnosis (98%; n=98/100), but only half (48%; n=48/100) had knowledge of their TB type, and 55% (n=6/11) of the adult TB patients with drug-resistant TB were aware of the drug resistance. In addition, we interviewed 71 HCWs, and more than one-third of HCWs (37%; n=26/71) were fully knowledgeable about paediatric TPT eligibility criteria, with 63% (n=45/71) unaware that HIV-infected children of all ages qualified for TPT after exposure
CONCLUSIONS: TPT provision in eligible child TB contacts in an urban district in SA was found to be suboptimal, especially for HIV-infected children. Instability in household configuration was an important reason for suboptimal TPT provision. Training of HCWs on paediatric TPT guidelines is required, together with knowledge sharing on TPT with the TB patients
Childhood tuberculosis (TB) preventive therapy (TPT) and active contact tracing, as per the South African (SA) national TB guidelines, form pivotal pillars in curbing childhood TB.[1] Previously published data documented suboptimal TPT implementation in the SA national TB control programme.[1] Further research on the uptake of paediatric TPT is therefore important, including on the current clinical practice of healthcare workers (HCWs), to better understand screening of child household contacts and subsequent TPT initiation in eligible children. This can assist in identifying operational challenges in the TPT programme implementation, resulting in improved service delivery.
The present study was aimed at identifying child household TB contacts (aged <5 years and/or HIV-infected) linked to infectious adult TB cases and documenting the proportion of these children having successfully initiated TPT. In addition, HCWs' and adult TB patients' knowledge on TPT, together with operational challenges in TPT provision, were determined.
Methods
This cross-sectional study used a mixed-method approach. Structured interviews were done with adult TB patients at primary healthcare clinics in the Tshwane subdistricts 3 and 4, the referral area of the Kalafong Provincial Tertiary Hospital. Tshwane District an urban district in the Gauteng Province of SA, has 7 service delivery regions and a population of 3 275 152.[2] In the community survey done in 2016, about half (51%) of the residents were employed and 16% of people were living in informal dwellings.[2] The present study was conducted between May and August 2017 and included 11 of the 13 clinics in the two subdistricts, excluding two clinics with very low numbers of adult TB patients. Data collection included socio-demographic data, TB- and HIV-related history, data on child contacts and TPT knowledge.
A convenience sample of adult TB patients was included in the study, with clinic visits for patient recruitment done by the principal investigator without any prior pre-arrangement with the clinic on the day of visit. All adult TB patients who were at the clinic on the day of study visit and who fulfilled the inclusion criteria were included in the study after providing informed consent. The inclusion criteria were adult TB cases (>18 years of age), with any type of TB, who were on current TB treatment and who consented to study inclusion. All patients were receiving TB treatment at the clinic prior to the study inclusion; therefore, eligible child TB contacts should already have been assessed and started on TPT. If eligible contacts were found not to be on TPT, the researchers assisted the local HCWs in tracing and subsequent TPT initiation.
The principal investigator interviewed each study participant individually using a self-developed questionnaire to assess elements of TPT knowledge. It included questions on childhood TB risk and subsequent management, as per SA TB guidelines (2013) relevant at the time. The questionnaire consisted of multiple-choice questions, with one correct answer among four options. HCWs and the adult TB patients were given the same set of six questions, with HCWs asked an additional 7th question on child TPT eligibility criteria. All interviews were done onsite at the clinics, either in a consulting room or another private space.
Additionally, adult TB patients were asked about the number of child contacts below the age of 5 years and/or HIV-infected children in their household. This information was then cross-checked in the onsite folders of the source case. Information on TB diagnosis and management, HIV diagnosis and management, and other relevant information were also obtained from the patient's folders, together with information on child contact identification, tracing and management.
The clinics' systems of TB screening and TPT initiation for affected child contacts were explored through onsite observations, focusing on its route of clinical decision making and also sought the effect of administrative processes, like the filing system, on the uptake of TPT among their child contacts.
Ethical approval was granted by the Research Ethics Committee at the University of Pretoria (ref. no. 54/2017). The Tshwane Research Committee and facility managers granted permission for the study to be conducted at the clinics, and TB patients as well as HCWs gave informed consent for study inclusion.
Descriptive statistics were utilised for statistical analysis. P-values were obtained using the Fishers exact test.
Results
We interviewed 100 adult index TB patients. The socio-demographic and medical factors of the adult index TB patients are shown in Table 1. The median (range) age of the adult TB patients was 39 (21 - 74) years.
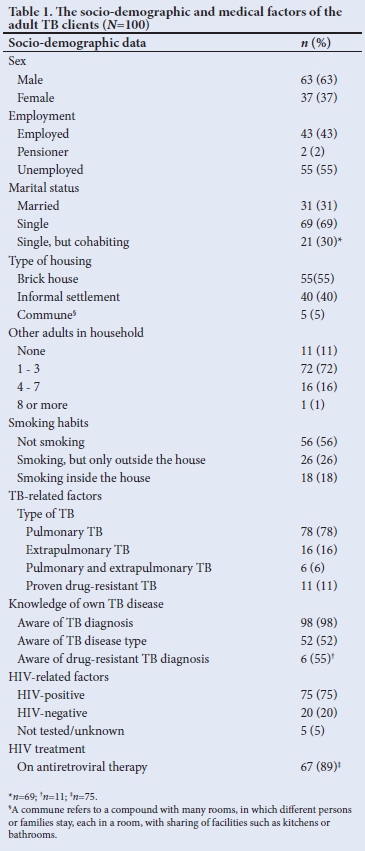
The clinic staff identified 15 eligible child contacts, and documented as such in the clinic folders, prior to the study, but only 6 of them were receiving TPT. An additional 13 child contacts were identified during the interviews. The coverage of TPT provision was therefore 21% (n=6/28). The 28 eligible children included 26 children <5 years of age, and two HIV-infected children, with no HIV-infected children included in the group previously identified by the clinic staff. None of the adult TB patients who participated in the present study and who had extrapulmonary TB only, were living with young (<5 years) or HIV-infected children. The majority of the interviewed adult TB patients were males (63%, n=63/100), but child contacts of female TB patients were more likely to be on TPT (40% for female patients v. 0% for male patients; p=0.002). No children had been diagnosed with TB and/or started on TB treatment.
In terms of households linked to the adult TB cases, 18 households had one child contact, while in five households, two children were linked to the adult source case, totalling 28 children from 23 households. In 15 of these households, the contacts were the TB patient's own children, while in the other 8 households, the contacts were children of other family members.
Missed opportunities for TPT provision included that adult patients had not been requested to bring their eligible child contacts to the health facility for assessment (30%; «=7/23). The remaining 16 patients (from the 23 affected households) had been requested by the HCWs to bring child contacts for assessment, but 44% (n=7/16) of the adults did not bring the children to the health facility. Changes in household composition and mobility of children between households were the most commonly provided reasons (57%; n=4/7). Other reasons included a private sector healthcare visit, with subsequent suboptimal management (n=1), while another TB patient believed it was the mother's responsibility to take the child for assessment and the other patient provided no reasons.
We interviewed 71 HCWs, representing all 11 clinics (5-7 HCWs/ clinic). The median (range) age of the HCWs was 32 (25 - 72) years and 93% (n=66/71) were female. Less than half of the interviewed HCWs (46%, n=33/71) were of the opinion that not all eligible child contacts were receiving TPT, most commonly ascribed to mobility of child contacts between households and/or geographical areas (36%; n=12/33).
The basic knowledge questions were mostly answered correctly by the HCWs (>80%), except for the question on childhood TPT eligibility criteria, which was only answered correctly by 37% of respondents (n=26/71). The most common wrong answer given to the eligibility criteria question was that all children were eligible for TPT (31%; n=22/71) and 25% (n=18/71) answered only children <5 years of age were eligible. The remaining HCWs answered that they did not know (8%, n=6/71). In addition, only 70% of the HCWs (n=50/71) correctly identified an immunocompromised state as being an additional risk factor for developing TB. Fig. 1 depicts the differences between the correct answers given by the HCWs and the adult TB patients. The adult TB patients incorrectly answered the question on underlying childhood risk factors for developing TB. Using a composite score, having a better TPT knowledge in adult TB patients did not significantly improve TPT access in their eligible children (p=1.000).

Onsite observations revealed non-standardised approaches to clinical and administrative management of childhood TPT provision. Two clinics were performing well, and together contributed 66% (n=4/6) to the actual total TPT coverage found in the present study. These were also the only two clinics where no new child contacts were identified during the study interviews and all the interviewed adult TB patients reported that they had been asked to bring the child contacts for assessment for possible TPT provision. In these clinics, the child TB contact folders were stored together with the adult TB patients' folders in a well-functioning filing system.
Discussion
Clinical assessment of child contacts of infectious adult TB cases significantly contributes to the identification of children who require TPT, as well as access to treatment for children with active TB, thereby reducing childhood mortality and morbidity[3] SA children have a disproportionately higher risk of TB due to the high TB burden, with paediatric TB cases higher than the global estimate of 10%, highlighting a need of effective preventive therapy. [4] The reduction of risk of progression from infection to disease in childhood TB contacts is well-documented,[1] particularly in cases of drug-susceptible TB and possible strains of low-level isoniazid resistance.[5] Awareness and implementation of the SA TPT guidelines in children to prevent TB is therefore of paramount importance. Previous studies done in Cape Town found that despite the recommendations by the World Health Organization and the SA National TB Programme being in place for >20 years, implementation of TPT in children was documented to be suboptimal.'
This current study focused on obtaining data on the coverage of paediatric TPT in the Tshwane District, which is a large urban SA health district with a large-scale adult HIV-TB co-epidemic. The coverage of TPT in eligible child contacts was found to be very low at 21% (n=6/28), very similar to the coverage previously documented in Cape Town (17%).[1] Additionally, in a recent systematic review of studies on child contact management in high TB-burden countries, 38% of studies reported TPT initiation rates of <50%.'61 More research is currently being conducted into the possibility of shorter courses of TPT, but the importance of identification of eligible children nevertheless will remain as the entry point for any such treatment simplification.
The poor detection of child contacts per index patient as found in this study is consistent with literature. The results from a study conducted in the Eastern Cape Province (SA), found 0.53 child contacts per index patient (n=261/491),[4] while we detected an average of 0.28 (n=28/100) eligible child contacts per index patient. The majority of patients in our study were male adult TB patients (63%; n=63/100). This finding is similar to the male predominance previously documented in Cape Town.[7] We found that children linked to male adult contacts were significantly less likely to have accessed TPT, most probably because women in the African context are tasked with the majority of child rearing.[8,9] TPT programmes therefore should place additional emphasis on active contact tracing of children linked to male TB patients.
The majority of the adult TB patients were HIV-positive and on antiretroviral treatment, indicating their regular interaction with the health system. Two adult TB patients indicated that they had HIV-positive child contacts, who were therefore eligible for TPT, but both had been missed for TPT provision prior to the present study. None of the adult TB patients had their child contact's HIV status recorded in their clinic folders, as the stationery did not routinely allow for this, neither did the stationery allow for documentation of details of child contacts <5 years of age. The majority of the adult TB patients did not know the HIV status of their children in the households, nor of its important link to TB prevention, indicating that the number of TPT-eligible HIV-positive children was likely underestimated in this study. This highlights the necessity of a strong partnership between the HIV treatment programme and the TB programme to ensure the identification of HIV-positive child contacts who are eligible for TPT.
A study done in Kenya[10] documented operational challenges hinging on the lack of a structured approach to identify and track TB-exposed children. Moreover, the Kenyan study showed that HCWs only sporadically asked index cases at the primary visit to bring child TB contacts to the clinic for screening.[10] In addition, only few index cases brought their children for screening and patient tracking was not done. In our study, this lack of structure in tracking and identifying TB-exposed children remained a challenge and mainly due to the mobility of the children between households. Transport difficulties, which included long travel times to clinics and movement of child contacts to intermittently live with extended family, were stated as the second major challenge pertaining to access of care in a previous systematic review[6] However, it was encouraging that our study participants were motivated to bring their child contacts for the required health assessment after they had been provided with information on the need for screening of child contacts. This is in line with results from a study done in 2017, which showed that targeted messages and educational materials improved families' awareness of child contact screening.[6]
We identified 13 additional child contacts during our study interviews, indicating that eligible children were missed despite adults accessing the required healthcare. Knowledge gaps of HCWs on crucial components of the national TB guidelines possibly contributed to suboptimal TPT provision. The fact that HCWs performed poorly on the question on childhood TPT eligibility criteria was a striking finding, with only 37% of respondents answering this crucial question correctly, pointing towards the well-documented critical need for continued in-service training of HCWs.[4] The difference in knowledge levels among the HCWs and adult TB patients, which we found in this study, suggests that improved knowledge sharing could potentially lead to adult TB patients who are better informed about TB and childhood TPT.
Our study also highlighted administrative gaps in optimising the management of child TB contacts at health facilities. Onsite observations done at the clinics with good TPT coverage showed the advantage of having a good filing system in place to enable smooth organisational flow, but even the clinics with good filing systems did not have individual clinic folders for the children. Documentation on the clinical assessment and TPT provision was found to be suboptimal. It was noted that in the clinics that used appointment cards for the child contacts had regular checking of adherence to TB testing and/or TPT protocol adherence. Dedicated stationery and onsite registers could potentially improve the clinical management and tracking of the child contacts, as recommended in other published studies.[6,10,1l] For the future, electronic records have great potential for better data management, although the implementation thereof comes with its own difficulties, including infrastructure needs and adoption of the new electronic systems by the staff.[12]
Study limitations
Limitations of this study include that we did not explore TPT initiation of HIV-positive child TB contacts during the children's HIV clinic visits to ascertain compliance with the TPT guidelines in that setting, and this is an area for future research. A non-standardised measurement tool was used in the form of multiple-choice questions; therefore, the influence of outliers and questions answered by chance potentially could have influenced the results. In addition, this study included a convenience sample obtained in two sub-districts in the Tshwane district in SA, potentially limiting generalisability. However, the research was done in a typical urban setting within the SA public healthcare system, in the context of clinical care provision according to the national TB guidelines, resulting in valuable and generalisable lessons.
Conclusion and recommendations
Child TB contact identification management remains challenging, as shown in this study in the Tshwane district of SA, especially in HIV-positive child contacts, calling for strong linkages between the HIV and TB programmes. Identification of eligible children is an essential step in the TB-prevention pathway, without which related concerns regarding optimisation of treatment duration, adherence, completion and efficacy are rendered ineffective.
Declaration. None.
Acknowledgements. None.
Author contributions. RTP and UDF conceptualised the study, analysed the data, wrote and revised the manuscript. Authors approved the final version of the manuscript for publication.
Funding. None.
Conflicts of interest. None.
References
1. Van Wyk SS, Reid AJ, Mandalakas AM, et al. Operational challenges in managing isoniazid preventive therapy in child contacts: A high burden setting perspective. BMC Public Health 2011;11(544):1-6. https://doi.org/10.1186/1471-2458-11-544 [ Links ]
2. Wazimap. City of Tshwane - profile data. Tshwane: CoT, 2016. https://wazimap.co.za/profiles/municipality-TSH-city-of-tshwane/ (accessed 25 July 2020). [ Links ]
3. Tadesse Y, Gebre N, Daba S, et al. Uptake of isoniazid preventive therapy among under-five children: TB contact investigation as an entry point. PLoS ONE 2016;11(5):1-11. https://doi.org/10.1371/journal.pone.0155525 [ Links ]
4. Black F, Amien F, Shea J. An assessment of the isoniazid preventive therapy programme for children in a busy primary healthcare clinic in Nelson Mandela Bay Health District, Eastern Cape Province, South Africa. S Afr Med ] 2018;108(3):217-223. https://doi.org/10.7196/samj.2018.vl08i3.12639 [ Links ]
5. Padayatchi N, Naidu N. Paediatric chemoprophylaxis for child contacts of patients with drug-resistant tuberculosis: Are current guidelines effective in preventing disease? S Afr Med J 2015;105(5):328-329. https://doi.org/10.7196%2FSAMJ.9500 [ Links ]
6. Szkwarko D, Hirsch-Moverman Y, Du Plessis L, Du Preez K, Carr C, Mandalakas AM. Child contact management in high tuberculosis burden countries: A mixed methods systematic review. PLoS ONE 2017;12(9)1-25. https://doi.org/10.1371/journal.pone.0182185 [ Links ]
7. Van Wyk SS, Hamade Η, Hesseling AC, Beyers N, Enarson DA, Mandalakas AM. Recording isoniazid preventive therapy delivery to children: Operational challenges. Int J Tuberc Lung Dis 2010;14(5):650-653. [ Links ]
8. Rutherford ME, Hill PC, Maharani W, et al. Risk factors for Mycobacterium tuberculosis infection in Indonesian children living with a sputum smear-positive case. Int J Tuberc Lung Dis 2012;16(12)1594-1599. https://doi.org/10.5588/ijtld.l2.0389 [ Links ]
9. Osman M, Hesseling AC, Beyers N, et al. Routine programmatic delivery of isoniazid preventive therapy to children in Cape Town, South Africa. Public Health Action 2013;3(3):199-203. https://doi.org/10.5588%2Fpha.13.0034 [ Links ]
10. Szkwarko D, Ogaro F, Owiti P, Carter EJ. Implementing a tuberculosis child contact register to quantify children at risk for tuberculosis and HIV in Eldoret, Kenya. Int J Tuberc Lung Dis 2013;3(3):209-213. https://doi.org/10.5588%2Fpha.l3.0018 [ Links ]
11. Rekha B, Jagarajamma K, Chandrasekaran V, Wares F, Sivanandham R, Swaminathan S. Improving screening and chemoprophylaxis among child contacts in India's RNTCP: A pilot study. Int J Tuberc Lung Dis 2013;17(2)163-168. https://doi.org/10.5588/ijtld.12.0415 [ Links ]
12. McCarthy B, Fitzgerald S, O'Shea M, et al. Electronic nursing documentation interventions to promote or improve patient safety and quality care: A systematic review. J Nurs Manag 2019;27(3):491-501. https://doi.org/10.1111/jonm.12727 [ Links ]
 Correspondence:
Correspondence:
R T Philip
roshneyt@gmail.com
Accepted 14 April 2021














