Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.16 no.1 Pretoria Abr. 2022
http://dx.doi.org/10.7196/sajch.2022.v16i1.1770
RESEARCH
An evaluation of challenges with the South African PMTCT HIV programme seen from the perspective of HIV-positive children admitted to the PICU
E E ElkhatialiI; P M JeenaII
IMB BCh, FCP (Paeds); Department ofPaediatric and Child Health, Nelson R Mandela School of Medicine, Faculty of Health Sciences, University of KwaZulu-Natal Durban, South Africa
IICert Paed Puim (SA), PhD; Department ofPaediatric and Child Health, Nelson R Mandela School of Medicine, Faculty of Health Sciences, University of KwaZulu-Natal Durban, South Africa
ABSTRACT
BACKGROUND: Mother-to-child transmissions (MTCT) accounts for 90% of the 370 000 new HIV-positive children, globally. Despite progress in the prevention of mother-to-child transmission (PMTCT) of HIV, children still acquire HIV infection
OBJECTIVE: To identify and describe the prevalence of maternal, infant and/or health system-related risk factors gleaned from the literature for HIV transmission in HIV-positive children admitted to the paediatric intensive care unit (PICU) at Inkosi Albert Luthuli Central Hospital (IALCH), Durban, South Africa
METHOD: A retrospective electronic chart review identifying all HIV-positive children under 2 years admitted to the PICU at IALCH between January 2017 and December 2019 was undertaken. Individual patient records were analysed using a standardised template
RESULTS: Of the 80 mothers and children with HIV enrolled in the present study, 38.8% (n=31/80) of mothers were diagnosed prior to pregnancy, 42.5% (n=34/80) were diagnosed during pregnancy (unsure when exactly transmission occurred), and 18.8% (n=15/80) of mothers were diagnosed after delivery. The median (range) time of antiretroviral treatment (ART) was 225 (30 - 365) days for mothers. More than half of mothers (56.3%, n=45/80) whose babies became HIV-positive had poor adherence to antiretroviral drugs (HIV viral load >1 000 copies/mL). An HIV-positive diagnosis in the children of these mothers occurred throughout infancy and early childhood, especially in the first 6 months (87.5%, n=70/80). A third of mothers practised mixed feeding. Health system deficiency, mainly via cancellation of tests without notifying healthcare workers, was typical in infants (33%; n=26/80) and mothers (68.8%, n=55/80). All others (100%) were not counselled about the importance of PMTCT and 93.8% of mothers were not counselled about the importance of follow-up. Almost all HIV-positive infants (95%, n=76) presented with severe respiratory illness, mainly severe acute respiratory distress syndrome (62.5%, n=50/80) and pneumonia with hypoxic respiratory failure (32.5%, n=26/80). The overall mortality of the cohort was 22.5% (n=18/80), and most deaths were associated with cytomegalovirus (CMV), Pneumocystis jirovecii pneumonia (PJP) or both (61.1%, n=11/18)
CONCLUSION: This present study confirmed that a new diagnosis of HIV positivity occurs throughout pregnancy and early childhood in infants. Poor adherence to ART in mothers and their infants, poor counselling, failure to attend antenatal and postnatal care, mixed feeding, and challenged laboratory services were common modifiable factors that need addressing
The HIV epidemic remains a major cause of morbidity and mortality in infants. Mother-to-child transmission (MTCT) accounts for >90% of all HIV-positive children globally, and it can occur during pregnancy, labour and/or breastfeeding.[1,2] UNAIDS estimated that the rate of HIV MTCT was 2% in South Africa (SA) in 2015, and a survey conducted in over 9 000 mothers and infants in 2012 -2013 showed an MTCT rate of 2.6% at 4 - 8 weeks in SA.[1,4,5] With the accurate implementation of prevention of mother-to-child transmission (PMTCT), HIV transmission reduced by 70% between 2009 and 2015.[6] A study by Goga et al.[7]showed that effective PMTCT reduced the risk of HIV MTCT remarkably. PMTCT interventions include the optimal use of antiretroviral treatment (ART) by the pregnant mother, appropriate labour and delivery practices, a short course of antiretroviral drugs (ARVs) for the baby and exclusive breastfeeding.[6,8]
HIV positivity in children continues to be a major public health challenge, with -120 000 children dying from HIV-related illnesses in 2016.[4] In 2017, 93% of HIV-positive women in sub-Saharan Africa had commenced ART, which resulted in a decreased rate of HIV MTCT from 18% in 2010 to 10% in 2017.[4] Early ART initiation for HIV-positive children enables a good prognosis.[9]
In SA, implementation of PMTCT services for HIV have been variable, so identifying gaps in the delivery of these services is crucial to eliminate vertical transmission and reduce paediatric infections.[10] The present study aimed to describe the prevalence of maternal, infant and/or health system-related risk factors gleaned from the literature for HIV transmission in HIV-positive children admitted to PICU at Inkosi Albert Luthuli Central Hospital (IALCH), Durban, SA.
Methods
This retrospective electronic chart review enrolled HIV-positive children admittedto thepaediatric intensive care unit (PICU) between January 2017 and December 2019. The researchers identified cases for enrolment by evaluating the electronic clinical and laboratory data of patients admitted to the PICU at IALCH. IALCH is a tertiary hospital (providing sub-specialist support to a regional hospital and requires the expertise of clinicians working as sub-specialists), and the PICU has 14 beds. On average, the unit admitted 450 cases each year, with an HIV infection rate of -15% for the period 2012 - 2016.
No cases are excluded from admission based on their HIV status. The average mortality rate for the unit during the same period was between 15% and 18% per annum. We captured data of HIV-positive children onto a Microsoft Excel spreadsheet using the SPSS software, version 25 (IBM Corp., USA). Mothers of enrolled subjects were contacted telephonically to obtain missing data on their risk for HIV transmission, maternal ART (type, duration, and adherence), HIV viral load (VL) testing, ART during labour and delivery, and ARV prophylaxis in infants and feeding practices after obtaining telephonic verbal informed consent. We captured possible reasons for non-adherence to recommended treatment and recorded failures of the health systems, including failure to follow departmental policy and lost blood results. We recorded the outcome of enrolled subjects, either as hospital discharges or as deaths and analysed the risk factors for HIV MTCT During the telephonic interview, follow-up outcome was recorded.
HIV-positive children <24 months of age admitted to the PICU during the study period (January 2017 - December 2019) were included in the analysis. HIV diagnosis was based on a positive HIV DNA PCR test using the COBAS AmpliPrep/COBAS TaqMan (CAP/CTM) HIV-1 Test version 2.0 (Roche Molecular Systems) and the Abbott's real-time PCR HIV-1 test, and any detectable HIV RNA VL used M200 real-time HIV-1 VL system.
Poor ART adherence was defined as mothers not taking medications daily (did not collect medication from a health facility or failure to take ART) on maternal interview and by inference from electronic laboratory records (high HIV VL and low CD4 count).
Poor counselling was defined as mothers reporting not being counselled on the importance of PMTCT on telephonic interviews or lack of records on counselling in the patient charts.
Mixed feeding was defined as infants on breast and formula feeding daily.
Expedited review of the proposal by the postgraduate committee of the University of KwaZulu-Natal and the UKZN Biomedical Research Ethics Committee was granted (ref. no. BE 551/81). The primary researcher contacted the parents telephonically with the aid of the nursing system to obtain information on adherence, counselling, and outcome with an understanding of the psychological challenges that might be experienced by the parents with the process. Parents were offered an opportunity to refuse the interview and allowed to refuse to answer any uncomfortable questions. Caution and sensitivity were taken into consideration during the interviews. All information was stored in a password-protected computer on a secure server, and full confidentiality and privacy were maintained.
Results
Of the 1 350 children admitted to the PICU at IALCH from 01 January 2017 to 31 December 2019, 5.9% (n=80) of children were HIV-positive, and of these children, 31% (n=25) already knew their HIV status before admission and 68.8% (n=55/80) were newly diagnosed on admission. Less than three-quarters (72.7%, n=40/55) of exposed mothers received PMTCT and 27.3% (n=15/55) were unexposed. More than two-thirds of children (68.8%, n=55/80) were females. The mean (range) age of the cohort was 4.5 (2 - 24) months. More than half of admitted children were HIV-negative (54.1%, n=730), 32.9% (n=444) were HIV-unexposed and 7.1% (n=96) had unknown HIV status. The risk factors for HIV MTCT were related to maternal, child and health system.
Maternal risk factors for HIV MTCT transmission in utero and post delivery
Of the 80 mothers of HIV-positive babies, 38.8% (n=31/80) were diagnosed prior to pregnancy, 42.5% (n=34/80) during pregnancy and 18.8% (n=15/80) after delivery. Upon confirmation of their HIV results, all mothers received a fixed drug combination of tenofovir, emtricitabine and efavirenz (Table 1). More than half of mothers (56.3%; n=45/80) whose babies became HIV-positive had poor adherence to ARV based on self-reporting from the mothers and HIV VL (Table 2).
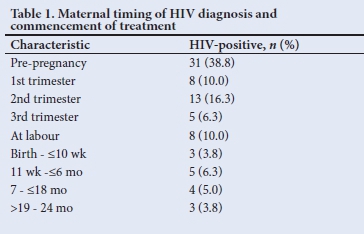
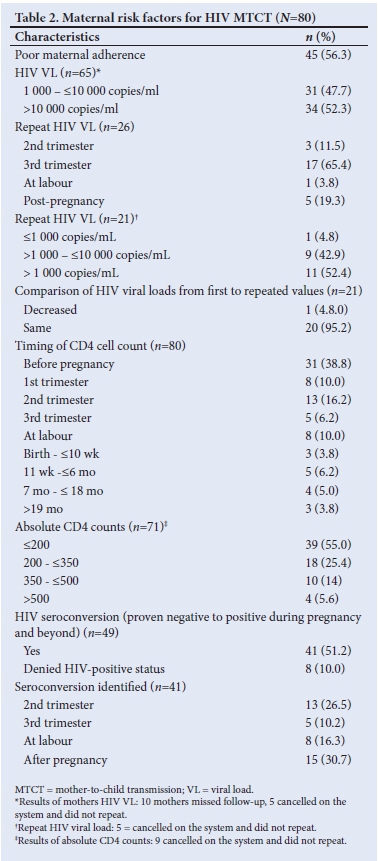
The HIV VLs among mothers (n=65/80) whose babies became HIV-positive were all >1 000 copies/mL, and CD4 counts were low (<200 cells/mm3). On repeat HIV VL testing, these remained unchanged (>1 000 copies/mL) in 20/65 of mothers (Table 2). Less than a tenth of HIV-positive mothers (6.3%, n=5/80) had TB, 1.2% (n=1/80) had syphilis, 7.5% (n=6/80) had pre-labour rupture of membrane (PROM) >18 hours, 2.5% (n=2/80) had depression and 3.8% (n=3/800) had vomiting of ARVs.
Maternal factors impacting on the transmission of intra- and postpartum HIV via MTCT
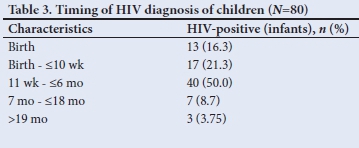
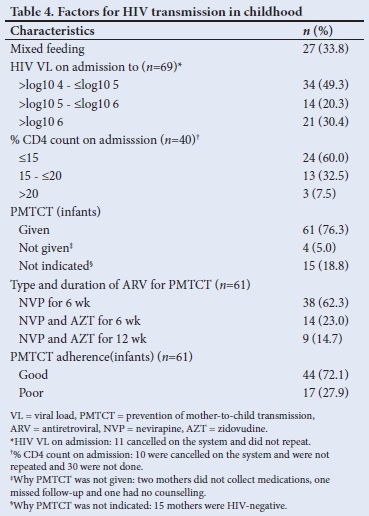
It was not so easy to distinguish early from late transmission, as a mother may have been infected during pregnancy and tested negative (false negative due to window period) on a rapid HIV test at delivery and only be newly diagnosed together with her infant a few months later while both were already infected during the pregnancy. The diagnosis of HIV positivity in children occurred throughout early childhood infancy but mainly in the first 6 months of life (87.5%; n=70/80) (Table 3). A third of mothers practised mixed feeding, and traditional scarifications were performed in 6 cases (7.5%) (Table 4). ARVs for PMTCT were administered to 93.8% (n=61/65) of HIV-exposed babies. Of these babies (37.7%; n=23/61) on ARV prophylaxis were regarded as high risk requiring dual ARV therapy and 27.9% (n=17/61) were non-adherent. Less than a tenth of babies of HIV-positive mothers (5%, n=4) diagnosed at birth did not receive ARVs for PMTCT, 18.8% (n=15) of mothers of HIV-positive babies were diagnosed after delivery and therefore did not receive ARV prophylactic therapy (Tables 3 and 4). Counselling of mothers on the need for PMTCT ARV therapy (n=65) and follow-up (n=75) was poorly performed in 100% and 93.8% of mothers, respectively.
Health system failure associated with MTCT
Health system failures associated with MTCT occurred both at maternal and infant level. The primary maternal failures in the health system were the cancellation of samples (HIV VL and CD4 count), results being recorded as pending at the laboratory level even during retrospective analysis, and the failure to present for follow-up. The main reason for failures for infants was the cancellation of samples (HIV VL and CD4 count) at the laboratory level, samples not being analysed and failure to follow-up.
Presentation and outcome of cases
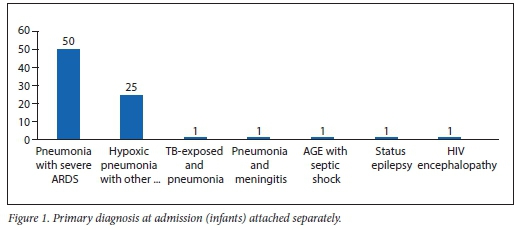
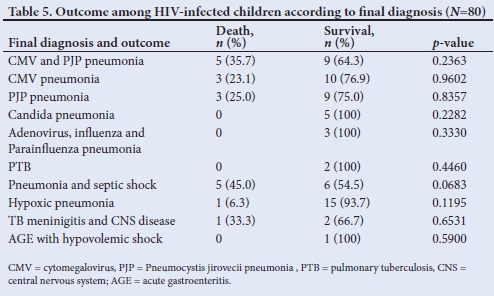
Almost all HIV-positive infants (95%, n=76) presented with severe respiratory illness, mainly severe acute respiratory distress syndrome (oxygen index >16, PaO2/FiO2 ratio <100 mmHg) (62.5%, n=50/80) and hypoxic pneumonia (severe pneumonia with type 1 respiratoryfailure) (31.3%, n=25/80) (Fig. 1). The overall mortality of the HIV-positive cohort admitted to PICU was 22.5% (n=18). Pneumonia associated with cytomegalovirus (CMV) (CMV VL >10 000 copies/mL), Pneumocystis jirovecii pneumonia (PJP) cultured from endotracheal aspiration or both accounted for 61.1% of the total mortality. Of the thirty-nine PJP/CMV co-infected cases, 11 died, giving a mortality rate of 28.2% (Table 5). While there were more females (25.5%) than males (11%) admitted with HIV infection, similar death rates were seen in both genders.
Discussion
The main finding of the present study is the continued transmission of HIV to pregnant women and their babies during pregnancy and the first 6 months of life despite the rollout of an effective PMTCT programme. We found a lack of adherence to maternal ART and infant prophylaxis, and failures in the health systems as causes for failed PMTCT. Other factors that contributed to MTCT of HIV were failure to initiate ART in children diagnosed HIV-positive and inadequate responses by healthcare professionals to high maternal HIV VL or cancellation of laboratory request for HIV testing, which should have resulted in enhanced adherence, ARV resistance testingor re-ordering of tests. Unfortunately, in the era of excellent PMTCT guidelines, infants who become HIV-positive are often disadvantaged by a mother who has poor health-seeking behaviour This often leads to the child having unsuppressed HIV VL.
These findings are concerning as they erode the gains made by the introduction of PMTCT, which was successful in reducing transmission rate below 2%, with treatment that reduces plasma HIV VL to undetectable levels.[11] Several studies have shown that in the absence of preventive measures, HIV MTCT had an incidence rate of 5 - 10% during pregnancy, 10 - 20% during labour and the postnatal period, but with the availability of preventive intervention, the risk can be as low as 1.3 - 4%.[12] HIV transmission can occur any time during pregnancy, delivery and up to 18 months postnatal period, with the greatest risks in pregnancy and first 6 months of life.[13]
The findings of the present study were similar to a study conducted in China[14] among 349 HIV-positive pregnant women whose HIV status was known before pregnancy(30.4%), during pregnancy (49.6%) and at or after childbirth (20.0%). Similarly, a study conducted in Cape Town, SA, showed that HIV MTCT was highest during the first 6 months postpartum, decreased between 6 and 12 months postpartum, with no infections occurring after 12 months.[15]
Globally, HIV awareness and knowledge about HIV transmission among young people has remained stagnant over the past 20 years, with only one in three people having appropriate knowledge about HIV transmission and prevention.[16] Previous studies have identified low maternal educational level, lack of knowledge about MTCT, poor access to health services and fear of Stigmatisation as obstacles to the uptake of HIV testing.[17] Improving access to HIV testing and counselling has been associated with an increase in knowledge of ones HIV status and a reduction in the overall risky behaviour in society.[18]
The other risk factors for HIV MTCT were opportunistic infections and PROM. During the antenatal and perinatal period, TB was a risk factor associated with increased risk of HIV transmission. It has been recommended that every pregnant woman diagnosed with HIV should be screened for TB, especially if their CD4 cell count is <50 cells/mm3.[19] In a Tanzanian study, pregnant women were found to have a higher rate of pulmonary TB, latent TB and other co-infections with HIV.[20] In a study by Minkoff et al, PROM due to chorioamnionitis was associated with a greater risk of vertical transmission of HIV, especially among mothers with low CD4 counts.[12]
The major postnatal risk factors for transmission included mixed feeding of infants, lack of adequate sampling or unavailability of results. The rate of mixed feeding in the present study was 33.8%, which is higher than what was observed in studies from Ethiopia (15.3%) and Kenya (18.2%).[21]
HIV transmission can be increased by 3 - 4-fold in infants with mixed feeding as compared with babies that were exclusively breastfed, especially among mothers with poor HIV VL suppression.[22] A study conducted in Nigeria[23] showed that the HIV transmission rate was higher in HIVexposed infants who were on mixed feeding (25.6%) than those who were exclusively breastfed (11.8%).
PICU admission and outcome for HIV-positive children failing PMTCT has not changed for the previous three decades, with pneumonia being the most common presenting problem. Pneumocystis jirovecii accounts for 10 - 49% of the aetiology of pneumonia in ART-naïve HIV-positive children in SA.[24] Morrow et al.[24] reported rapid progress of HIV disease in infected infants. CMV pneumonitis has higher prevalence rates in HIV-infected andexposed infants admitted to the PICU, where it is noted to have worse outcomes as compared with HIV-uninfected children.[26] A study by Rabie et al.[27]found a high incidence of TB among HIV-positive infants before the implementation of combination ART with high morbidity and mortality in the TB and HIV co-infected children in the absence of ART.
Study limitations
Our study had several limitations, which resultedfrom the retrospective methodology. The completeness of clinical records was inadequate and attempts to address this were made by telephone interviews. The measurement of adherence and counselling is notoriously difficult, especially when there is an adverse outcome of the infants becoming HIV-positive, and this could have resulted in bias. However, attempts to measure adherence and counselling were undertaken by direct interview and through inference from the laboratory results.
Conclusion
In conclusion, this study showed continued HIV transmission during pregnancy and post delivery in mothers and babies. This burden can be avoided by addressing modifiable factors related to maternal, children and healthcare system (i.e. poor adherence to ART in mothers and their infants, poor counselling and failure to attend antenatal care and postnatal clinics, mixed feeding and deficient laboratory services over a prolonged period). Simple interventions to address these deficiencies must be enforced. The new PMTCT guidelines of birth and 10 weeks HIV PCR testing, nevirapine for 6 weeks, third-trimester maternal PCR testing and treatment are designed to catch new infections in pregnancy at delivery and postpartum.
Declaration. This study was done in partial fulfilment of requirements for the MMed degree.
Acknowledgements. We would like acknowledge Dr M Archary for reviewing this manuscript, Mr Ebenzer Ogunsakin for statistrical analysis, Mrs Leora Sewnarain for assistance with formatting and language editing. IALCH for granting permission to conduct the study.
Author contributions. EEE conceptualised and designed the study, collected, and analysed data and wrote the drafting manuscript. PI supervised the study and revised the manuscript. All authors approved the final version of manuscript for publication.
Funding. None.
Conflicts of interest. None.
References
1. Peltzer K, Weiss SM, Soni M, et al. A cluster randomised controlled trial of lay health worker support for prevention of mother to child transmission of HIV (PMTCT) in South Africa. AIDS Res Therapy 2017;14(1):61. https://doi.org/10.1186%2Fsl2981-017-0187-2 [ Links ]
2. Bohlius J, Maxwell N, Spoerri A, et al. Incidence of AIDS-defining and other cancers in HIV-positive children in South Africa: Record linkage study. Pediatr Infect Dis J 2016;35(6):e164. https://doi.org/10.1097%2FlNE0000000000001117 [ Links ]
3. Ngeno B, Rogers B, Mbori-Ngacha D, Essajee S, Hrapcak S, Modi S. Understanding the uptake of prevention of mother-to-child transmission services among adolescent girls in Sub-Saharan Africa: A review of literature. Int J Adolesc Youth 2020;25(1):585-598. https://doi.org/10.1080/02673843.2C19.1699124 [ Links ]
4. The Joint United Nations Programme on HIV and AIDS (UNAIDS). Ending AIDS: Progress towards the 90-90-90 targets. Global AIDS update. Geneva: UNAIDS, 2017. https://www.unaids.org/sites/default/nles/media_asset/Global_AIDS_update_2017_en.pdf [ Links ]
5. Mnyani C, Tait CL, Peters RP, et al. Implementation of a PMTCT programme in a high HIV prevalence setting in Johannesburg, South Africa: 2002-2015. Southern Afr J HIV Med 2020;21(1):7. https://doi.org/10.4102%2Fsajhivmed.V21i1.1024 [ Links ]
6. Ramoshaba R, Sithole SL. Knowledge and awareness of MTCT and PMTCT post-natal follow-up services among HIV infected mothers in the Mankweng region, South Africa. Open AIDS J 2017;11:36. https://doi.org/10.2174/1874613601711010036 [ Links ]
7. Goga AE, Dinh TH, Jackson DJ, et al. Population-level effectiveness of PMTCT option A on early mother-to-child (MTCT) transmission of HIV in South Africa: Implications for eliminating MTCT. J Glob Health 2016;6(2):020405. https://doi.Org/10.7189/jogh.6.020405 [ Links ]
8. Okoko NA, Owuor KO, Kulzer JL, et al. Factors associated with mother-to-child transmission of HIV despite overall low transmission rates in HIVexposed infants in rural Kenya. Int J STD AIDS 2017;28(12):1215-1223. https://doi.org/10.1177/0956462417693735 [ Links ]
9. Feucht UD, Meyer A, Thomas WN, Forsyth BW, Kruger M. Early diagnosis is critical to ensure good outcomes in HIV-infected children: Outlining barriers to care. AIDS Care 2016;28(l):32-42. https://doi.org/10.1080/09540121.2015.1066748 [ Links ]
10. Pellowski J, Wedderburn C, Stadler JAM, et al. Implementation of prevention of mother-to-child transmission (PMTCT) in South Africa: Outcomes from a population-based birth cohort study in Paarl, Western Cape. BMJ Open 2019;9(12):e033259. https://doi.org/10.1136/bmjopen-2019-033259 [ Links ]
11. World Health Organization. Global guidance on criteria and processes for validation: Elimination of mother-to-child transmission of HIV and syphilis, 2nd edition. Geneva: WHO, 2017. https://www.who.int/reproductivehealth/publications/emtct-hiv-syphilis/en/. [ Links ]
12. Yah CS, Tambo E. Why is mother to child transmission (MTCT) of HIV a continual threat to newborns in sub-Saharan Africa (SSA). J Infect Public Health 2019;12(2):213-223. https://doi.Org/10.1016/j.jiph.2018.10.008 [ Links ]
13. Robb L, Walsh C, Nel M. Knowledge, perceptions and practices of HIV-infected mothers regarding HIV and infant feeding. S Afr J Clin Nutrition 2020;33(l):23-29. https://doi.org/10.1080/16070658.2018.1503810 [ Links ]
14. Tang L, Zhang C, Gao S, Wang Z, Miao H, Xia J. Epidemiological characteristics of HIV infected pregnant women and exposed infants in Guangdong province. 2014-2017. Zhonghua liu xing bing xue za zhi 2019;40( 11):1392. https://doi.org/10.3760/cma.j.issn.0254-6450.2019.11.010 [ Links ]
15. Le Roux SM, Abrams EJ, Nguyen KK, Myer L. HIV incidence during breastfeeding and mother-to-child transmission in Cape Town, South Africa. AIDS 2019;33(8):1399-1401. https://doi.org/10.1097/qad.0000000000002224 [ Links ]
16. The Joint United Nations Programme on HIV and AIDS (UNAIDS). Global AIDS update 2019 - communities at the centre: Defending rights, breaking barriers, reaching people with HIV services. Geneva: UNAIDS, 2019. https://www.unaids.org/sites/default/nles/media_asset/2019-global-AIDS-update_en.pdf [ Links ]
17. Ejigu Y Tadesse B. HIV testing during pregnancy for prevention of mother-to-child transmission of HIV in Ethiopia. PLoS One 2018; 13(8):e0201886. https://doi.org/10.1371%2Fjournal.pone.0201886 [ Links ]
18. De Dieu Anoubissi J, Gabriel EL, Nde CK, et al. Factors associated with risk of HIV-infection among pregnant women in Cameroon: Evidence from the 2016 national sentinel surveillance survey of HIV and syphilis. PLoS One 2019;14(4):e0208963. https://doi.org/10.1371/journal.pone.0208963 [ Links ]
19. Duff P. Prevention of opportunistic infections in women with HIV infection. Clin Obstet Gynaecol 2019;62(4):816-822. https://doi.org/10.1097/grf.0000000000000483 [ Links ]
20. Gebreegziabiher D, Adane K, Abebe M. A survey on undiagnosed active pulmonary tuberculosis among pregnant mothers in Mekelle and surrounding districts in Tigray, Ethiopia. Int J Mycobacteriol 2017;6(l):43-46. https://doi.org/10.4103/2212-5531.201889 [ Links ]
21. Andare N, Ochola S, Chege P. Determinants of infant feeding practices among mothers living with HIV attending prevention of mother-to-child transmission Clinic at Kiambu level 4 hospital, Kenya: A cross-sectional study. Nutr J 2019;18(1):64. https://doi.org/10.1186/sl2937-019-0490-y [ Links ]
22. Ejara D, Mulualem D, Gebremedhin S. Inappropriate infant feeding practices of HIV-positive mothers attending PMTCT services in Oromia regional state, Ethiopia: A cross-sectional study. Int Breastfeeding J 2018;13( 1):37. https://doi.org/10.1186/sl3006-018-0181-x [ Links ]
23. Abebe ZZ, Mengistu MY, Gete YK, Worku AG. Mother-to-child HIV transmission among infants born to HIV-positive women in Amhara National Regional State, Ethiopia. Recent Adv Biol Med 2020;6(2020):11866. [ Links ]
24. Morrow BM, Samuel CM, Zampoli M, Whitelaw A, Zar HJ. Pneumocystis pneumonia in South African children diagnosed by molecular methods. BMC Research Notes 2014;7(1):26. https://doi.org/10.1186/1756-0500-7-26 [ Links ]
25. Ellington SR, Clarke KE, Kourtis AP. Cytomegalovirus infection in human immunodeficiency virus (HlV)-exposed and HIV-infected infants: A systematic review. J Infect Dis 2016;213(6):891-900. https://doi.org/10.1093/infdis/jiv549 [ Links ]
26. Jeena PM, Govender K, Parboosing R, Adhikari M. The significance of cytomegalovirus in children with pneumonia admitted for mechanical ventilation. Int J Tuberc Lung Dis 2017;21(12):1230-1236. https://doi.org/10.5588/ijtld.l7.0026 [ Links ]
27. Rabie H, Goussard P. Tuberculosis and pneumonia in HIV-infected children: An overview. Pneumonia 2016;8(1): 19. https://doi.org/10.1186/s41479-016-0021-y [ Links ]
 Correspondence:
Correspondence:
E Elkhatiali
emhemedl981@gmail.com
Accepted 1 March 2021














