Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Child Health
versión On-line ISSN 1999-7671
versión impresa ISSN 1994-3032
S. Afr. j. child health vol.15 no.3 Pretoria sep. 2021
http://dx.doi.org/10.7196/SAJCH.2021.v15.i3.1799
RESEARCH
Outcomes of extremely low-birthweight neonates at a tertiary hospital in the Western Cape, South Africa: A retrospective cohort study
G M MusiimeI; L G LloydII; M McCaulIII; N van ZylIV; S L HolgateV
IFCPaeds (SA); Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIFCPaeds (SA), Cert Neonatol; Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIIPhD; Division of Epidemiology and Biostatistics, Department of Global Health, Stellenbosch University, Cape Town, South Africa
IVMB ChB; Department of Paediatrics, Tygerberg Hospital, Cape Town, South Africa
VFCPaeds (SA), Cert Neonatology Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
ABSTRACT
BACKGROUND. Neonates of extremely low birthweight (ELBW; <1 000 g) have the highest neonatal mortality in South Africa (SA). Objective. To describe the morbidity and mortality of ELBW neonates treated at a tertiary hospital in SA.
METHODS. This was a retrospective cohort study including all live-born ELBW neonates treated at Tygerberg Hospital between 1 January and 31 December 2016. Data were extrapolated from a prospectively collected neonatal database and patient records. Multiple logistic regression and survival analysis were performed to identify risk factors of mortality.
RESULTS. The sample included 256 neonates. The following morbidities were recorded: respiratory distress syndrome (83.2%); bronchopulmonary dysplasia (8.2%); intraventricular haemorrhage (34.5%); periventricular leukomalacia (0.6%); necrotising enterocolitis (10.5%); and retinopathy of prematurity (31.2%). The survival-to-discharge rate was 63.3%. Cause of death was documented as extreme prematurity in 41% of the inpatient deaths. Birthweight was a significant predictor of mortality (hazard ratio 0.99; 95% confidence interval 0.992 - 0.999). Of the 162 neonates who survived until discharge, 11 died following discharge.
CONCLUSION. Morbidity and mortality rates remain high among ELBW neonates. To improve survival, resources need to be allocated to neonatal resuscitation, surfactant therapy and increasing availability of intensive-care beds.
Neonatal deaths are a leading cause of mortality worldwide, accounting for an estimated 2.7 million deaths globally in 2017.[1]In South Africa (SA), access to intensive care for neonates of extremely low birthweight (ELBW; <1 000 g) has been limited over the past decade because of resource constraints.[2 3] They have the highest mortality, require delivery room resuscitation more frequently than infants weighing more than 1 000 g and have a significantly higher risk of mortality following resuscitation.[4, 5] Data from tertiary centres in Johannesburg and Cape Town demonstrate an improvement in survival of ELBW neonates over time[2, 6-10] and the majority of preterm neonates survive without neurodevelopmental impairment (NDI) although intrauterine and neonatal insults are associated with long-term disability.[11,12] ELBW is a risk factor for NDI[12] However, in three studies that have assessed long-term outcomes of ELBW neonates in SA, a small number of neonates presented with NDI (although loss to follow-up was significant).[19, 10, 13]
If the sustainable development goal of reducing neonatal mortality to 12 per 1 000 live births by 2030 is to be realised in low- and middle-income countries,[14] urgent attention must be focused on reducing the mortality of ELBW neonates. There are limited data evaluating the long-term outcomes of ELBW neonates in SA and few studies focus specifically on the short-term outcomes of these neonates. In addition, because of variations in outcomes over time and between regions, and increased access to nasal continuous positive airway pressure (CPAP) and surfactant therapy in recent years, morbidity and mortality data cannot be easily transposed. There is a need for recent data focusing on both long- and short-term outcomes of ELBW neonates in SA to guide forward planning, resource allocation and policy development with a view to optimising outcomes and decreasing mortality.
The purpose of the present study was to describe the morbidity and mortality of ELBW neonates treated at Tygerberg Hospital (TBH) in 2016. The primary outcomes were neonatal morbidities and all-cause mortality. The secondary outcomes were risk factors for neonatal mortality.
Methods
Study design and population
This was a retrospective cohort study. All live-born neonates with a birthweight <1 000 g and admitted to TBH between 1 January 2016 and 31 December 2016 were included in this study. Outborn neonates admitted to TBH after day 28 of life were excluded. This cohort was identified from data entered into a prospectively collected neonatal database. Data were supplied by admitting doctors using a standardised form and then verified and entered into a password-protected database by a consultant.
Standard of care
All infants were managed according to hospital guidelines at the time. Neonates were admitted to the neonatal high-dependency ward where nasal-prong oxygen, nasal CPAP and surfactant were administered according to the hospital protocol. Owing to resource constraints, infants with a birthweight <750 g and gestational age (GA) <26 weeks received surfactant selectively. Surfactant would be given to these neonates if antenatal care attendance was adequate, if at least two doses of antenatal betamethasone were administered to the mother at least 12 hours apart within seven days prior to delivery, and if prolonged resuscitation following delivery was not required.
Neonates requiring intensive care would be considered for admission to the neonatal intensive care unit (NICU) once they reached a corrected GA of 28 weeks and weighed at least 1 000 g. Under exceptional circumstances and depending on bed availability, infants not meeting these criteria were considered for admission to the NICU on a case-by-case basis. This included neonates born to mothers with a history of recurrent pregnancy losses or following in vitro fertilisation, provided antenatal care attendance was adequate and a complete course of betamethasone had been administered to the mother prior to delivery. Neonates deemed to have poor prognosis by the attending neonatologist, specifically neonates with severe congenital anomalies, severe birth asphyxia, grade 4 intraventricular haemorrhage or requiring extensive resuscitation following delivery, were not eligible for NICU admission irrespective of birthweight or GA.
GA was routinely determined by early ultrasound, defined as an ultrasound undertaken at less than 24 weeks' gestation as per hospital protocol. Foot length at birth was used as an indicator when early ultrasound was not available.[15]
Prevention of mother-to-child transmission (PMTCT) of HIV entailed initiation of single or dual antiretroviral prophylaxis after delivery according to risk stratification. A blood sample was obtained for HIV polymerase chain reaction analysis at birth and three-drug antiretroviral therapy was initiated in the event of HIV seroconversion. Exclusive breastfeeding was recommended as per hospital guidelines and donor breastmilk was used, with informed maternal consent, if there was insufficient expressed breastmilk.
Outcomes
Definitions of outcomes were adapted from the Vermont Oxford Network (VON) manual of operations.[16] However, bronchopulmonary dysplasia (BPD) was not categorised by the VON manual and was therefore defined as treatment with supplemental oxygen of >21% for more than 28 days[17] Hypothermia was defined as core body temperature <36.5 °C and presumed sepsis was defined as clinical signs of generalised infection, elevated inflammatory markers or an elevated cerebrospinal fluid protein count with pleocytosis in the absence of a positive blood or cerebrospinal fluid culture. At TBH, neonates fulfilling these criteria are treated with antibiotics for at least 5 days at the discretion of the neonatologist.
Data on maternal demographics included antenatal care attendance, syphilis, HIV and administration of antenatal steroids and magnesium sulphate. Delivery details included: place of birth; indication for delivery; multiple gestation; mode of delivery; Apgar score at 1, 5 and 10 minutes; resuscitation details; and temperature on admission to the neonatal unit. Neonatal information included birthweight and GA. Birthweight below the tenth centile for the GA, as plotted on the growth curves of Fenton, was classified as small for gestational age (SGA)[18]
Data on neonatal morbidity included details of: congenital anomalies; the need for respiratory support after birth and its duration; NICU admission and duration; patent ductus arteriosus; BPD; respiratory distress syndrome and surfactant administration; intraventricular haemorrhage (all grades); cystic periventricular leukomalacia; necrotising enterocolitis and its management (all stages, including suspicion); focal intestinal perforation and its management; retinopathy of prematurity (ROP) and its management (all stages included, as diagnosed by an ophthalmologist); sepsis (early-onset, late-onset and presumed sepsis); HIV prophylaxis; and HIV status at birth and subsequent follow-up. Mortality was classified according to the primary cause of death.
Data management and statistical methods
Data were extracted from the database and additional data were obtained from patient records. Descriptive analysis was performed in SPSS.P9] Results are presented as counts with proportion (n, %), 95% confidence intervals (CI) and medians with the associated interquartile range (IQR). Survival analysis, simple regression analysis and multiple Cox regression analysis were used to identify risk factors for mortality in STATA;P°]results are presented as a Kaplan-Meier curve, /'-values and hazard ratios (HRs) and associated 95% CIs. For purposes of the analysis, it was assumed that cases lost to follow-up were not all due to death during the study period. A subgroup analysis was performed to identify risk factors for inpatient mortality.
Sample size was estimated a priori using the OpenEpi sample size calculator for cohort studies[21] A value of 25% was used as the percentage of exposed cases with outcome, which represents the proportion of ELBW infants with respiratory distress syndrome as the primary cause of death; this figure was derived from published SA data.[3]The total sample size derived from the calculator was increased by 20 for each additional cause of death as classified in the study. A minimum sample size of 118 was required to evaluate factors associated with mortality with 80% power and a 95% CI, assuming an equal number of exposed and unexposed cases.
Ethical considerations
Ethical approval for the study was obtained from the Human Research Ethics Committee of Stellenbosch University (ref. no. S18/05/103). A waiver of individual informed consent was granted by the committee. Permission to conduct research at TBH was obtained from the Western Cape provincial government.
Results
Study population
A total of 256 ELBW neonates were admitted to TBH during the study period, of whom 16 (6.3%) were outborn. Close to two-thirds of the admitted neonates (n=162, 63.3%) survived until hospital discharge, of whom 11 subsequently died. Of the remaining 151 (59%) survivors, 65 (43%) had complete one-year follow-up data and 86 (57%) were lost to follow-up.
Characteristics, morbidity and interventions
The characteristics of the cohort and perinatal interventions are presented in Table 1. Neonatal morbidity and treatment are summarised in Table 2. Data on cranial ultrasound were not available for 88 neonates (34.4%). A total of 170 neonates survived to four weeks post delivery or for 31 - 32 weeks after conceptual age and were therefore eligible for ROP screening; 28 of these neonates (16.5%) were not screened.
Mortality and cause of death
Mortality according to GA is presented in Table 3. Mortality for neonates at the limit of viability at birth (23 - 26 weeks' gestation) is presented in Table 4. Causes of inpatient deaths were as follows: extreme prematurity (41%); sepsis (10.5%); respiratory distress syndrome (9.5%); necrotising enterocolitis (9.5%); pulmonary haemorrhage (6.7%); focal intestinal perforation (1%); intraventricular haemorrhage (1%); aspiration (1%); infective endocarditis (1%); and infected ventriculoperitoneal shunt (1%). In 18.1% of deaths, the cause of death was classified as unknown; in these cases a final cause of death was not documented in the patient records. The stillbirth rate during the study period was 501 per 1 000 births (still and live births included).
Risk factors for mortality and survival analysis
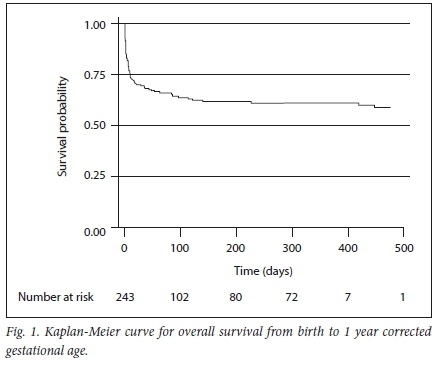
Results of multiple Cox regression analysis for mortality risk factors are shown in Table 5. Included variables were selected on the basis of known association with mortality or statistically significant association with mortality on univariate analysis (p<0.05). Not all potential confounding variables were included in the model because of the small sample size. Antenatal care was not included owing to high attendance (91.8%). NICU admission was not included, as only 11.3% of the cohort was admitted to the NICU. Birthweight was a significant predictor of mortality (HR 0.99; 95% CI 0.992 - 0.999). A subgroup analysis was performed to identify risk factors for inpatient mortality and the same variables were incorporated into the model. Birthweight was the only significant predictor of mortality (HR 0.99; 95% CI 0.992 - 0.995). A Kaplan-Meier curve for overall survival from birth to one year corrected GA is shown in Fig. 1. The incidence of mortality was two per 1 000 days, assuming not all infants lost to follow-up died during the specified study period.
Discussion
This retrospective cohort study provides updated data on the outcomes of ELBW neonates cared for at TBH. The overall survival-to-discharge rate was 63.3% for ELBW neonates born in 2016. This is lower than the 74.8% rate reported at TBH between 2007 and 2009.P1 Although the mean birthweight and GA were similar during these periods, the earlier higher survival rate may, in part, be ascribed to differences in access to intensive care; neonates weighing 750 g or of 26 weeks' GA were eligible for NICU admission at TBH between 2007 and 2009.
The NICU admission criteria were subsequently revised because of a considerable increase in the number of neonates admitted over time, with a corresponding shortage of NICU bed space. In addition, the higher patient load and turnover noted in 2016, in the absence of a substantial increase in bed capacity or staffing due to resource constraints, conferred an increased risk of infection and therefore mortality.[19]
In comparison with other tertiary centres in SA, the survival-to-discharge rate reported here (63.3%) is slightly higher than the rate reported for the Charlotte Maxeke Johannesburg Academic Hospital (52.4%) in 2013[6]and that reported for Grey's Hospital (49.5%) in KwaZulu-Natal,[20] but lower than the rate at Groote Schuur Hospital (68%) between 2003 and 2005.P1 In comparison with other upper middle-income countries, this survival rate is lower than rates reported in Jamaica and the South American Neocosur Network (five countries), and similar to rates reported in Thailand and Malaysia.[21, 24] These differences may partly be explained by variations in access to NICUs, with the countries with lower mortality rates using lower birthweight and GA criteria for NICU admission and mechanical ventilation. Most deaths occurred in the first three days of life, similar to global mortality data.[25] The most commonly listed cause of death in the present study was extreme prematurity, consistent with national data.[26] The rates of sepsis, respiratory distress syndrome, intraventricular haemorrhage, necrotising enterocolitis and BPD were similar to previous data from TBH and other centres in SA[2, 8, 9] There were insufficient data on ROP from other studies in SA for comparison.
Birthweight was a statistically significant risk factor for inpatient mortality in this study, with increasing birthweight associated with reduced risk of death consistent with global and national data[8, 25] Other factors known to be associated with inpatient mortality were not found to be of statistical significance in the regression analysis. This is likely a consequence of the sample size being fairly small.
The results of this study have highlighted areas of good clinical practice. Antenatal care attendance was high and the majority of HIV-exposed infants received adequate PMTCT treatment as per hospital guidelines; for the one neonate who tested HIV positive, there was no PMTCT or antenatal care attendance.
The findings of this study also point to areas for improving clinical practice. Of the eligible neonates, 28 were not screened for ROP. Although there is only one ophthalmologist who conducts ROP screening at TBH, efforts should be made to optimise screening to prevent long-term visual impairment. Cranial ultrasound was not conducted in 88 cases; of these, 50 died in the first three days of life. At TBH, cranial ultrasounds are performed by the radiology department, neonatology fellows and neonatologists. It is therefore not always possible to obtain cranial ultrasounds for neonates in high-dependency wards within 72 hours of delivery. Training of junior doctors to perform cranial ultrasounds may be an option for improving coverage. Administration of antenatal magnesium sulphate for neuroprotection and antenatal steroids should be optimised.
This study highlights further research questions. There is a need for a well-designed, adequately powered prospective cohort study evaluating short- and long-term outcomes of ELBW infants. This would facilitate a comparative analysis of morbidity and mortality rates. As the weight criteria for eligibility for surfactant therapy and NICU admission have been revised downwards since 2016, it is possible that there may be a corresponding decline in the mortality rate. The development of a regional electronic database and audit tool may facilitate the collection of comprehensive long-term outcome data for ELBW neonates.
Study limitations
The most significant limitation of this study was that, owing to the retrospective design, data were incomplete. In addition, verification of final diagnosis was not possible and diagnoses were provided by different caregivers with varying levels of training and experience. There was also a risk of confounding, as for the purpose of survival analysis it was assumed that not all cases lost to follow-up were due to death during the specified study period. The study was not sufficiently powered to incorporate all potentially confounding variables into the regression analysis.
Conclusion
Mortality and morbidity rates remain high among extremely preterm infants. To improve survival, resources need to be allocated to neonatal resuscitation, surfactant therapy, nasal CPAP and increased availability of NICU beds. Antenatal care attendance and PMTCT coverage are high; however, ROP screening and administration of antenatal magnesium sulphate and antenatal steroids should be optimised to minimise morbidity.
Declaration. None.
Acknowledgements. None.
Author contributions. GMM wrote the manuscript with inputs from LGL, SLH, MM and NVZ. Data were extracted from the neonatal database by LGL and additional data were collected by GMM and NVZ. Statistical analysis was performed by GMM and MM. All authors read and approved the final draft before submission.
Funding. None.
Conflicts of interest. None.
References
1. Hug L, Alexander M, You D, Alkema L. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: A systematic analysis. Lancet Glob Health 2019;7(6):e710-e720. https://doi.org/10.1016/s2214-109x(19)30163-9 [ Links ]
2. Kirsten GF, Kirsten CL, Henning PA, et al. The outcome of ELBW infants treated with NCPAP and InSurE in a resource-limited institution. Pediatrics 2012;129(4):e952-e959. https://doi.org/10.1542/peds.2011-1365d [ Links ]
3. Kalimba EM, Ballot D. Survival of extremely low-birth-weight infants. S Afr J Child Health 2013;7(1):13-16. https://doi.org/10.7196/sajch.488 [ Links ]
4. Pattinson R, Rhoda N. Saving Babies 2012-2013: Ninth report on perinatal care in South Africa for the PPIP group. https://www.ppip.co.za/wp-content/uploads/Saving-Babies-2012-2013.pdf (accessed 24 May 2020). [ Links ]
5. Ballot DE, Agaba F, Cooper PA, et al. A review of delivery room resuscitation in very low birth weight infants in a middle income country. Matern Health Neonatol Perinatol 2017;3(1):9. https://doi.org/10.1016/j.resuscitation.2017.05.004 [ Links ]
6. Ballot DE, Chirwa T, Ramdin T, et al. Comparison of morbidity and mortality of very low birth weight infants in a Central Hospital in Johannesburg between 2006/2007 and 2013. BMC Pediatr 2015;15:20. https://doi.org/10.1186/s12887-015-0337-4 [ Links ]
7. Velaphi SC, Mokhachane M, Mphahlele RM, Beckh-Arnold E, Kuwanda ML, Cooper PA. Survival of very-low-birth-weight infants according to birth weight and gestational age in a public hospital. S Afr Med J 2005;95(7):504-509. [ Links ]
8. Ballot DE, Chirwa TF, Cooper PA. Determinants of survival in very low birth weight neonates in a public sector hospital in Johannesburg. BMC Pediatr 2010;10:30. https://doi.org/10.1186/1471-2431-10-30 [ Links ]
9. Adu-Boahene A. Outcome of extremely low birth weight infants in a resource limited setting. MD Thesis. New Haven: Yale University, 2011. https://elischolar.library.yale.edu/ymtdl/1534 (accessed 24 May 2020). [ Links ]
10. Thompson CM, Buccimazza SS, Webster J, Malan AF, Molteno CD. Infants of less than 1250 grams birth weight at Groote Schuur Hospital: Outcome at 1 and 2 years of age. Pediatrics 1993;91(5):961-968. [ Links ]
11. Blencowe H, Lee ACC, Cousens S, et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res 2013;74 Suppl 1(Suppl 1):17-34. https://doi.org/10.1038/pr.2013.204 [ Links ]
12. Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: A systematic review. Lancet 2012;379(9814):445-452. https://doi.org/10.1016/j.yobg.2012.06.155 [ Links ]
13. Cooper PA, Sandler DL. Outcome of very low birth weight infants at 12 to 18 months of age in Soweto, South Africa. Pediatrics 1997;99(4):537-544. https://doi.org/10.1542/peds.99.4.537 [ Links ]
14. United Nations. Sustainable Development Goals: 17 goals to transform our world. https://www.un.org/sustainabledevelopment (accessed 24 May 2020) [ Links ]
15. Van Wyk L, Smith J. Postnatal foot length to determine gestational age: A pilot study. J Trop Pediatr 2016;62(2):144-151. https://doi.org/10.1093/tropej/fmv093 [ Links ]
16. Vermont Oxford Network. Vermont Oxford Network 2018 Manual of Operations: Part 2. Data Definitions and Infant Data Forms. Vermont: VON, 2017. [ Links ]
17. Davidson LM, Berkelhamer SK. Bronchopulmonary dysplasia: Chronic lung disease of infancy and long-term pulmonary outcomes. J Clin Med 2017;6(1):4. https://doi.org/10.3390/jcm6010004 [ Links ]
18. Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr 2003;3(1):13. https://doi.org/10.1186/1471-2431-3-13 [ Links ]
19. Dramowski A, Aucamp M, Bekker A, Mehtar S. Infectious disease exposures and outbreaks at a South African neonatal unit with review of neonatal outbreak epidemiology in Africa. Int J Infect Dis 2017;57:79-85. https://doi.org/10.1016/j.ijid.2017.01.026 [ Links ]
20. Luthuli NP, McKerrow NH. Short-term outcomes of infants with an extremely low birth weight in a resource-limited neonatal intensive care unit, Grey's Hospital, KwaZulu-Natal. S Afr J Child Health 2019;13(3):120-124. https://doi.org/10.7196/sajch.2019.v13i3.1575 [ Links ]
21. Ministry of Health, Malaysia. Report of the Malaysian National Neonatal Registry. Kuala Lumpur: Minsitry of Health, 2012. [ Links ]
22. Trotman H, Lord C. Outcome of extremely low birthweight infants at the University Hospital of the West Indies, Jamaica. West Indian Med J 2007;56(5):410-413. [ Links ]
23. Sritipsukho S, Suarod T, Sritipsukho P. Survival and outcome of very low birth weight infants born in a university hospital with level II NICU. J Med Assoc Thai 2007;90(7):1323-1329. [ Links ]
24. Fernandez R, D'Apremont I, Dominguez A, Tapia JL, Red Neonatal Neocosur. [Survival and morbidity of very low birth weight infants in a South American neonatal network]. Arch Argent Pediatr 2014;112(5):405-412. [https://doi.org/10.5546/aap.2014.405] [ Links ]
25. Lawn JE, Blencowe H, Oza S, et al. Every Newborn: Progress, priorities, and potential beyond survival. Lancet 2014;384(9938):189-205. https://doi.org/10.1016/s0140-6736(14)60496-7 [ Links ]
26. Rhoda N, Velaphi S, Gebhardt GS, Kauchali S, Barron P. Reducing neonatal deaths in South Africa: Progress and challenges. S Afr Med J 2018;108(3a):9. https://doi.org/10.7196/samj.2017.v108i3b.12804 [ Links ]
 Correspondence:
Correspondence:
G M Musiime
gm.musiime@gmail.com
Accepted 13 December 2020














