Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.15 n.3 Pretoria Sep. 2021
http://dx.doi.org/10.7196/SAJCH.2021.v15.i3.1771
RESEARCH
Evaluating maternal characteristics and DNA polymerase chain reaction birth testing of neonates born with HIV in a KwaZulu-Natal referral hospital - missed opportunities?
S AbushkiwaI; R SinghII; K L NaidooIII
IFCPaeds (SA); Department of Paediatric and Child Health, Nelson R Mandela School of Medicine, Faculty of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IIFCPaeds (SA), Cert Neonatology (SA); King Edward VIII Hospital, KwaZulu-Natal Department of Health, Durban, South Africa
IIIFCPaeds (SA), PhD; King Edward VIII Hospital, KwaZulu-Natal Department of Health, Durban, South Africa
ABSTRACT
BACKGROUND. Congenital HIV infection has declined in South Africa with an expanded programme to decrease perinatal HIV transmission. Understanding the challenges within this programme can identify opportunities for improvements. There is an opportunity with birth HIV DNA polymerase chain reaction testing to expedite very early treatment of neonates born with HIV in referral hospitals.
OBJECTIVES. This study was aimed at describing the characteristics of mothers of neonates born with HIV as well as the evaluation of the access and availability of birth HIV DNA PCR results within a referral hospital.
METHOD. This study was a retrospective chart review of all documented neonates born with HIV delivered between 1 January 2017 and 30 June 2019 at King Edward VIII Hospital, Durban, KwaZulu-Natal. The birth HIV DNA PCR results provided through institutional programmes were analysed, and the clinical characteristics of mothers of neonates born with HIV were described.
RESULTS. Review of hospital-based records, during the 30-month study period, identified 6 902 (40.02% of17 243 total live births) birth HIV DNA PCR tests having been done. During the sample period, documented positive, rejected or indeterminate results were analysed. These results indicated only 37 (0.54%) of known HIV-exposed neonates who were tested, to have a documented positive birth HIV DNA PCR result. Fifty-nine neonates had either indeterminate or rejected results. Negative HIV DNA PCR results could not be verified. Analysis of the mothers of neonates with documented HIV-positive results indicated high mean viral loads of these mothers (52 343 copies/mL) and most were diagnosed in the current pregnancy or at delivery.
CONCLUSION. Review of the characteristics of mothers of neonates born with HIV within a public referral hospital reiterates that late HIV diagnosis is common in this high-risk group. Birth HIV DNA PCR results may fail to adequately identify all positive neonates timeously for very early initiation of antiretroviral treatment.
South Africa (SA) has among the highest antenatal HIV seroprevalence rates in the world, ranging from 16.9 to 37.4 %, with KwaZulu-Natal (KZN) being the province with the highest rates.[1,2] Specific areas in KZN, particularly the urban sections of eThekwini (Durban municipality), have antenatal HIV seroprevalence rates of 40.1%.[3] Programmes to decrease perinatal HIV transmission have evolved over the past few years in SA to achieve improved accessibility and have been relatively successful in decreasing the paediatric HIV disease burden.[4] Repeat antenatal HIV testing, simplification to single-dose antiretroviral treatment (ART), and widespread availability of viral load testing are some of the changes that have occurred.[5] Challenges, however, remain with various components in these programmes.[4] At-risk mothers generally book late, or are newly diagnosed with acute HIV infections and often have poor social and economic support.[6,7]
Maternal viral load is a strong independent predictor of the risk of transmission, regardless of timing.[8] Maternal factors that are associated with suppression of HIV viral load, however, can vary across geographical socioeconomic areas, and these factors need to be further understood within the operational context of large, busy referral hospitals.
Referral hospitals generally cater to high-risk deliveries, who are either referred or who bypass primary care facilities. Follow-up of HIV care and treatment, including reviewing birth HIV test results, is often compromised in the referral hospital as mothers and neonates are down-referred back to primary care facilities.
Much of the public health guidelines is primarily based on evidence from clinical trials, and translating evidence from these trials into operational practice is often difficult, particularly in resource-limited settings.[9] Evaluation of prevention programmes at an operational level is therefore needed.
An additional key change in the SA programme is the early diagnosis of neonates who acquired HIV by vertical transmission.[5,10] For very early initiation of ART in neonates born with HIV, it would be ideal that this occurred as soon as possible after delivery at an institution that has the capacity to provide neonatal ART treatment. Most SA women deliver within institutions, and this affords the opportunity where, if HIV DNA polymerase chain reaction (PCR) testing is available at birth and results accessed timeously, very early ART treatment can be commenced prior to discharge from these institutions.[11,12] At referral hospitals, the very early treatment of neonates born with HIV can be expedited as the capacity to treat neonates with ART is present. Very early initiation of ART has been shown to significantly reduce the risk of mortality in children with the additional possibility of reducing the size of the latent reservoir in infected children.[13] The evaluation of the access to birth HIV DNA PCR testing and timeous availability of results at the referral hospital, to which mothers are often transferred for largely obstetric reasons, need to be understood.
The primary objective of this study was thus to describe the maternal characteristics of neonates born with HIV within a referral hospital in an area with very high antenatal HIV seroprevalence. A secondary objective of the study was to evaluate whether birth HIV DNA PCR testing within referral hospitals can support the very early initiation of ART in neonates identified with HIV, by determining access to and availability of HIV DNA PCR results.
Methods
This study was a retrospective cohort study utilising data from in-hospital HIV results, inpatient records and laboratory provided results. The study was conducted in King Edward VIII Hospital (KEH) in Durban, KZN, SA. KEH is a regional/tertiary referral hospital that drains a large population base of both urban and semi-urban communities with a high HIV seroprevalence.
Ethics approval from the University of KwaZulu-Natal (UKZN) Biomedical Research Ethics Committee (BREC ref no. BE 415/18), and gatekeeper permission from the KZN Department of Health and KEH was obtained prior to data collection and verification. The initial protocol indicated a study period of two years (1 January 2017 -31 December 2018) but, following the small sample size of identified HIV-positive neonates, ethics approval was extended till June 2019.
For the period of the study from 1 January 2017 to 30 June 2019, the total number of live births was obtained from ward-based records in two areas (labour ward and neonatal unit). Data for HIV-exposed deliveries were also obtained from these two separate ward-based records based on admission data. These obtained data were then cross-referenced with institutional records maintained by the facility information officer who sources institutional data through a different mechanism that is based on patient records. The number of HIVexposed deliveries was also further cross-referenced with the National Health Laboratory System (NHLS) laboratory records.
The birth HIV DNA PCR results of all known HIV-exposed infants were reviewed. These birth HIV DNA PCR results are provided weekly to the institution by the NHLS. Three sources were then used to identify and verify these positive results. These were neonatal records, institutional HIV treatment clinic records, and NHLS records. The data from all three sources were cross-referenced. Results from the NHLS, which are provided each week, include a list of positive, indeterminate and rejected samples. Once results were cross-referenced and checked, they were then entered into a Microsoft Excel spreadsheet and coded for either positive, indeterminate or rejected results.
The birth PCR DNA results done at or soon after the birth of known HIV-exposed neonates were included. Repeat samples, or samples taken after inpatient admission to the institution, were not evaluated. This inclusion criterion was specifically used to simulate the operational practice in most referral hospitals and keeping with the second objective of the study, to evaluate the access to and availability of the first birth HIV DNA PCR test and results.
The negative birth HIV DNA PCR results of all HIV-exposed neonates could not be cross-referenced from NHLS monthly records as only positive, indeterminate and rejected results are provided. All negative results are either recorded within patient charts prior to discharge on a patient-to-patient basis if available, and these records are hospital records only. Only these results were used as a means to determine negative results.
It must be noted that if weekly results provided by the NHLS did not include a positive, rejected or indeterminate result, and the neonate and mothers were eligible for discharge, that mother could have been discharged to verify the assumed negative result at a local clinic. However, all positive, rejected and indeterminate results are actively traced and called back to the institution through the institutional HIV treatment clinic.
This limitation in validating negative results is noted and, additionally, some neonates would not have been tested post delivery as their mothers could have tested HIV-negative but still been in the window period.
The primary investigator then reviewed all the inpatient records of all known neonates confirmed to have been diagnosed with HIV, as well as their mothers' records during this period. Information regarding maternal antenatal bookings, number of antenatal visits, demographic and socioeconomic information, date of HIV diagnosis and HIV viral load, CD4 cell count and haemoglobin (Hb) were obtained. For all known positive neonates', information on the mode of delivery, gender, need for additional medical care (need for oxygen and ventilation), as well as the average length of stay and mortality, was also extracted from inpatient records and entered into to the Excel spreadshseet (Microsoft Corp., USA).
Data analysis
Captured data were analysed by a statistician using the Statistical Package for Social Science version 25 (IBM Corp., USA). Descriptive statistics, such as frequencies and percentages, were used to summarise categorical variables. Central tendency and dispersion of data were measured using means and standard deviations for normally distributed variables and medians and interquartile ranges for skewed variables.
Results
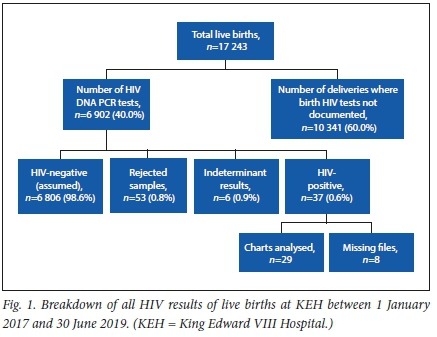
There were 17 243 live births documented in the institution during the study period, A total of 6 902 initial DNA birth PCR tests were done during this same period, indicating 40.02% of live births were identified as HIV exposed at birth and for which testing was done in this study period. A total of 6 750 mothers were documented to be HIV-positive in this same study period. The additional 152 DNA birth PCR tests done as compared with the number of known HIV-exposed mothers were accounted for by multiple pregnancies, inter-hospital neonatal transfers and immediate repeat testing within the seven-day period.
During the study period, only 37 birth HIV DNA PCR results were identified as positive (0.54% of all HIV-exposed deliveries), 6 (0.09%) were indeterminate, and 53 results were categorised as rejected. The rejected results included samples that were rejected owing to technical errors in the collection (compromised sample cards because of sampling techniques used, or insufficient/incorrect information provided on the requisition forms).
All of these results were available by day 7 following delivery. The number of negative results could not be verified within these 7 days as only positive, indeterminate and rejected results were provided by the NHLS weekly as part of the programme. Negative results had to be sourced on a patient-to-patient basis and entered into the patient folders.
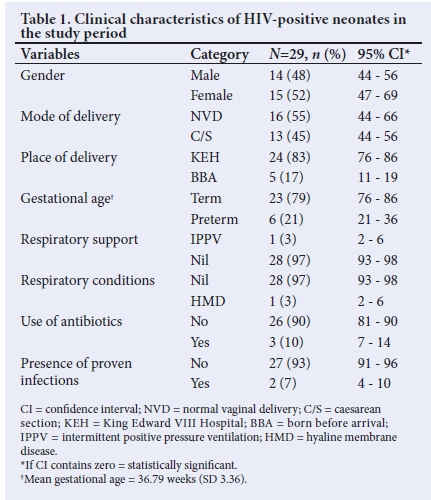
The data extracted from the 37 neonates with positive HIV DNA results with available records have been tabulated in Table 1.
Maternal characteristics
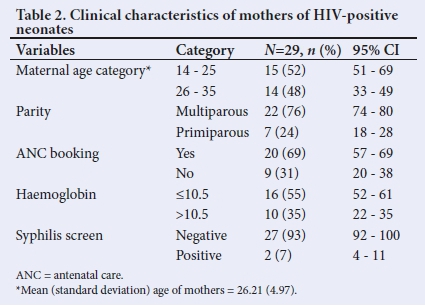
Table 2 displays the clinical characteristics of mothers of neonates with positive HIV DNA results. Over half (55.2%) of the mothers of the neonates with documented positive HIV DNA PCR results had a documented anaemia during pregnancy.
Maternal viral load and CD4 counts
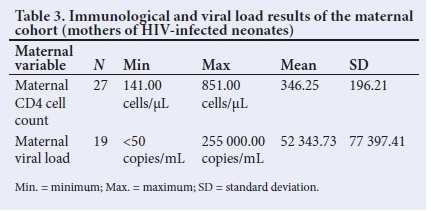
In this study, maternal viral loads were high in the majority of the mothers (65.5%), with a mean viral load of (52 343.7 copies/mL). Ten of the mothers had an unknown viral load. The mean CD4 count of this cohort of mothers was 346.
Table 3 shows the mean values of the CD4 cell counts and viral loads of this cohort of mothers.
Maternal socioeconomic characteristic
There were gaps in information on the socioeconomic characteristics of mothers. In the available results, 51.7% of mothers in this cohort were unemployed, and 41.4% indicated the absence of a supportive partner. Table 4 lists the maternal and socioeconomic characteristics of the mothers of HIV-positive neonates in the study period.
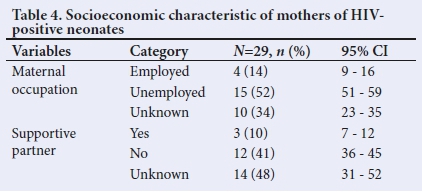
Discussion
In the present study, based at a referral hospital with a high HIV antenatal seroprevalence, the characteristics of documented mothers of neonates born with HIV and the available birth HIV DNA PCR test results during the study period are described.
Maternal diagnosis and awareness of HIV status
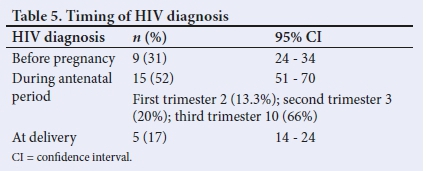
The late diagnosis of HIV is common in mothers of HIV-infected neonates.[13] In a 2017 survey, 39.2% of HIV-positive pregnant women nationally were unaware of their HIV-positive status prior to their first antenatal visit.[4] The present study found that most (68.9%) mothers of neonates born with HIV had not been diagnosed with HIV prior to antenatal care or delivery.
The average viral load of antenatal booked mothers in SA was noted to be <1 000 copies/mL in keeping with targets to ensure that 90% of patients on ART have complete viral suppression.[6] While the present study did not compare characteristics between the mothers of HIV-infected neonates and those of HIV-uninfected neonates, the mean viral load of mothers analysed was much higher than that of the average antenatal booked SA mothers.[13,14]
The high maternal viral loads in the study cohort and the timing of maternal HIV diagnosis with the majority of mothers diagnosed post conception possibly suggests that this high-risk group could have large numbers of acute HIV infections as documented in previous studies.[15] Acute HIV infections are associated with significantly elevated HIV burden in the plasma and genital secretions, thus increasing the likelihood of transmission.[16] In a study in Botswana, 43% of all vertical transmissions were estimated to be transmissions from mothers who seroconverted after their first antenatal screening visit.[17] Moreover, Trepka et al.[18] found that late diagnosis of maternal HIV infection appeared to be primarily the result of acute maternal infections and inadequate prenatal care. Johnson et al.[19] found that maternal seroconversion during late pregnancy and breastfeeding contributed significantly to the paediatric HIV burden, and needed greater attention in HIV prevention programmes.
Anaemia is a common problem among antenatal attendees in an SA urban population. However, in the majority, it is mild normocytic and normochromic (Hb 10 - 10.9 g/dL).[20] Tunkyi et al.[20] found that anaemia was 2.5 times higher among HIV-infected pregnant women than among uninfected ones. In the present study, maternal documented anaemia (Hb <10.5 g/dL) was noted in more than half of the mothers whose babies were diagnosed with HIV.
The results of this study highlight that despite the availability of an accessible perinatal HIV prevention programme, there remains a cohort of women who are unaware of their HIV status prior to pregnancy. This remains an important opportunity that should not be missed. Preconception and antenatal HIV testing remain essential to identify HIV-infected women who need to start antiretrovirals (ARVs) both to decrease the risk of perinatal HIV transmission and to improve maternal health.
Social determinants of disease, including income level, employment and partner support, play an important role in improving maternal health outcomes in mothers living with HIV.[21] In the present study, the trends supported the view that high-risk mothers who are transmitting HIV have significant social factors that impede care. Partner support is an issue that needs to be looked at within antenatal care, and studies have shown that there are improved HIV outcomes among couples via HIV counselling and testing.[22]
Neonatal characteristics
Preterm birth (born before 37 completed weeks) is associated with a higher risk of perinatal HIV transmission, especially for those born before 33 weeks.[23] A meta-analysis in 2015 showed that HIV-infected women were at higher risk of having low-birthweight infants or preterm delivery infants compared with uninfected women. Such associations did not change significantly over time or were not significantly affected by the usage of ARVs, which were not found to decrease risks of either low birthweight or preterm delivery associated with maternal HIV exposure.[24] In the present study, 20% of the study population were premature neonates with a median gestational age of 36 weeks. As no comparisons were made in this study, this is an important factor that needs to be evaluated further in larger studies.
Birth HIV DNA PCR testing
The relatively low percentage of documented positive results accessed post delivery could partially reflect a decreasing perinatal HIV transmission seen in SA.[25,26] However, cause for concern is the number of rejected, indefinite results and the inability to verify all negative results post discharge from a referral hospital. With the use of maternal ARV treatment, lower HIV viral loads in infants with true infection could potentially compromise an assay's ability to detect HIV in infected infants, leading to false-negative or indeterminate test results.[27,28] Indeterminate test results require repeat testing as it has been documented that half of those with an indeterminate result were HIV-positive.[29] We postulate that in this study, several neonates born with HIV were probably not identified within a week post delivery. The failure thus to identify all neonates born HIV positive could indicate a missed opportunity to start very early ART in a vulnerable population.
Recommendations
High-risk mothers who are unaware of their HIV status need to be prioritised in antenatal care and repeat HIV testing of negative pregnant mothers must be prioritised.
HIV PCR DNA testing needs optimising with improvement in techniques of sampling, urgent repeat testing for indeterminate results, and timeous access to both positive and negative birth results ideally prior to discharge of HIV-exposed neonates and their mothers from referral institutions.
Study limitations
This study was specifically based on data that were accessed from institutional recording systems and hence reflected the challenges inherent with these systems of data keeping. The results might have been affected by the retrospective nature of the chart review, as 8 files of 37 postive neonates were missing.
The characteristics of mothers of HIV-positive, -negative,-exposed and -unexposed neonates were not compared.
The present study evaluated perinatal HIV prevention programme using available birth HIV DNA PCR results. This represented in utero transmissions, and hence the true transmission rates need to be evaluated by by assessing HIV DNA PCR results at 10 weeks postnatally and post breastfeeding cessation.
The study was confined to one hospital, which receives referrals, mainly, and does not reflect the district hospitals, primary healthcare clinics and midwife-run obstetric units where follow-up is more clearly defined.
Conclusion
The present study identifies two areas where opportunities exist for improvement in programmes related to the care of mothers and neonates infected with HIV in referral hospitals. The findings suggest that many high-risk mothers who transmit HIV to their neonates within the context of an accessible and expanded perinatal HIV prevention programme had high HIV viral loads and were unaware of their HIV diagnosis prior to being pregnant. The contribution of acute HIV infections to poor HIV control requires further investigation. The study further found that birth HIV DNA PCR testing may not identify all possible HIV-positive neonates timeously. While it holds an opportunity to fast-track early initiation of ART, this finding suggests that HIV DNA PCR testing at birth needs urgent review. This is especially true for referral hospitals, where availability of records and access to timeous results if often difficult/ problematic.
Declaration. None.
Acknowledgements. The authors express their gratitude to Mr Ogunsakin Ebenezer for his assistance with the statistical analyses and Mrs Leora Sewnarain for assistance with formatting and language review.
Author contributions. SA: study design, data collection, data analysis and drafting the manuscript. RS: supervision of the entire work, study design and manuscript review. KLN: study design, data analysis and manuscript review.
Funding. None.
Conflicts of interest. None.
References
1. Kharsany AB, Frohlich JA, Yende-Zuma N, et al. Trends in HIV prevalence in pregnant women in rural South Africa. J Acquir Immune Defic Syndr 2015;70(3):289. [ Links ]
2. Jackson DJ, Dinh T-H, Lombard CJ, Sherman GG, Goga AE. An approach for evaluating early and long term mother-to-child transmission of HIV (MTCT) in low and middle income countries: A South African experience. BMC Infect Dis 2019;19(1):1-8. [ Links ]
3. Nozulu N, Gaede BM. Antiretroviral initiation of pregnant women and antenatal care booking practices in eThekwini District, KwaZulu-Natal, South Africa. Afr J Prim Health Care Fam Med 2018;10(1):e1-e9. https://doi.org/10.4102%2Fphcfm.v10i1.1606 https://dx [ Links ]
4. Goga AE, Dinh T-H, Jackson DJ, et al. First population-level effectiveness evaluation of a national programme to prevent HIV transmission from mother to child. S Afr J Epidemiol Comm Health 2014;69(3):240-248. https://doi.org/10.1136/jech-2014-204535 [ Links ]
5. South African National Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults, 2015. https://sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf (accessed 17 April 2020). [ Links ]
6. Woldesenbet SA, Kufa T, Barron P, et al. Viral suppression and factors associated with failure to achieve viral suppression among pregnant women in South Africa. AIDS 2020;34(4):589-597. https://doi.org/10.1097/qad.0000000000002457 [ Links ]
7. Stinson K, Myer L. Barriers to initiating antiretroviral therapy during pregnancy: A qualitative study of women attending services in Cape Town, South Africa. Afr J AIDS Res 2012;11(1):65-73. https://doi.org/10.2989/16085906.2012.671263 [ Links ]
8. Luzuriaga K, Mofenson LM. Challenges in the elimination of pediatric HIV-1 infection. N Engl J Med 2016;374(8):761-770. https://doi.org/10.1056/NEJMra1505256 [ Links ]
9. Jónasson JO, Deo S, Gallien J. Improving HIV early infant diagnosis supply chains in sub-Saharan Africa: Models and application to Mozambique. Operations Res 2017;65(6):1479-1493. https://doi.org/10.1287/opre.2017.1646 [ Links ]
10. Abebe ZZ, Mengistu MY, Gete YK, Worku AG. Mother-to-child HIV transmission among infants born to HIV-positive women in Amhara National Regional State, Ethiopia. Recent Adv Biol Med 2020;6(2020):11866. https://doi.org/10.18639/RABM.2020.963114 [ Links ]
11. Khupakonke S, Beke A, Amoko DHA. Maternal characteristics and birth outcomes resulting from births before arrival at health facilities in Nkangala District, South Africa: A case control study. BMC Pregnancy Childbirth 2017;17(1):401. https://doi.org/10.1186/s12884-017-1580-5 [ Links ]
12. Doctor HV, Nkhana-Salimu S, Abdulsalam-Anibilowo M. Health facility delivery in sub-Saharan Africa: Successes, challenges, and implications for the 2030 development agenda. BMC Public Health 2018;18(1):765. https://doi.org/10.1186/s12889-018-5695-z [ Links ]
13. Goetghebuer T, Haelterman E, Marvillet I, et al. Vertical transmission of HIV in Belgium: A 1986-2002 retrospective analysis. Euro J Pediatrics 2009;168(1):79. https://doi.org/10.1007/s00431-008-0717-y [ Links ]
14. Coll O, Hernandez M, Boucher C, et al. Vertical HIV-1 transmission correlates with a high maternal viral load at delivery. J Acquir Immune Defic Syndr 1997;14(1):26-30. https://doi.org/10.1097/00042560-199701010-00005 [ Links ]
15. Drake AL, Wagner A, Richardson B, John-Stewart G. Incident HIV during pregnancy and postpartum and risk of mother-to-child HIV transmission: A systematic review and meta-analysis. PLoS Med 2014;11(2):e1001608. https://doi.org/10.1371/journal.pmed.1001608 [ Links ]
16. Mayaphi SH, Martin DJ, Quinn TC, et al. Detection of acute and early HIV-1 infections in an HIV hyper-endemic area with limited resources. PLoS ONE 2016;11(10):e0164943. https://doi.org/10.1371/journal.pone.0164943 [ Links ]
17. Lu L, Legwaila K, Motswere C, Smit M, Jimbo W, Creek T, eds. HIV incidence in pregnancy and the first post-partum year and implications for PMTCT programs, Francistown, Botswana, 2008. Abstract, 16th Conference on Retroviruses and Opportunistic Infections. Montreal, Canada 8 - 11 February 2009. [ Links ]
18. Trepka MJ, Mukherjee S, Beck-Sagué C, et al. Missed opportunities for preventing perinatal transmission of human immunodeficiency virus, Florida, 2007 - 2014. South Med J 2017;110(2):116. https://doi.org/10.14423/smj.0000000000000609 [ Links ]
19. Johnson LF, Stinson K, Newell M-L, et al. The contribution of maternal HIV seroconversion during late pregnancy and breastfeeding to mother-to-child transmission of HIV. J Acquir Immune Defic Syndr 2012;59(4):417. [ Links ]
20. Tunkyi K, Moodley J. Prevalence of anaemia in pregnancy in a regional health facility in South Africa. S Afr Med J 2015;106(1):101-104. https://doi.org/10.7196/samj.2016.v106i1.9860 [ Links ]
21. Kwambai TK, Dellicour S, Desai M, et al. Perspectives of men on antenatal and delivery care service utilisation in rural western Kenya: A qualitative study. BMC Pregnancy Childbirth 2013;13(1):134. https://doi.org/10.1186/1471-2393-13-134 [ Links ]
22. Darbes LA, van Rooyen H, Hosegood V, et al. Uthando Lwethu ('our love'): A protocol for a couples-based intervention to increase testing for HIV: A randomised controlled trial in rural KwaZulu-Natal, South Africa. Trials 2014;15(1):64. https://doi.org/10.1186/1745-6215-15-64 [ Links ]
23. Levin C, le Roux DM, Harrison MC, Tooke L. HIV transmission to premature very low birth weight infants. Pediatr Infect Dis J 2017;36(9):860-862. https://doi.org/10.1097/inf.0000000000001611 [ Links ]
24. Xiao P-L, Zhou Y-B, Chen Y, et al. Association between maternal HIV infection and low birth weight and prematurity: A meta-analysis of cohort studies. BMC Pregnancy Childbirth 2015;15(1):246. https://doi.org/10.1186%2Fs12884-015-0684-z [ Links ]
25. Dunning L, Kroon M, Fourie L, Ciaranello A, Myer L. Impact of birth HIV-PCR testing on the uptake at follow-up early infant diagnosis (EID) services in Cape Town, South Africa. Pediatr Infect Dis J 2017;36(12):1159. https://doi.org/10.1097/inf.0000000000001677 [ Links ]
26. Bisschoff C, Coulon J, Isaacs Z, et al. HIV testing at birth: Are we getting it right? South Afr J HIV Med 2019;20(1):1-5. https://doi.org/10.4102/sajhivmed.v20i1.951 [ Links ]
27. Mazanderani AH, Technau K-G, Hsiao N-Y, Maritz J, Carmona S, Sherman GG. Recommendations for the management of indeterminate HIV PCR results within South Africa's early infant diagnosis programme. South Afr J HIV Med 2016;17(1):451. https://doi.org/10.4102%2Fsajhivmed.v17i1.451 [ Links ]
28. Luo R, Boeras D, Broyles LN, et al. Use of an indeterminate range in HIV early infant diagnosis: A systematic review and meta-analysis. J Acquir Immune Defic Syndr 2019;82(3):281-286. https://doi.org/10.1097/qai.0000000000002104 [ Links ]
29. Mazanderani AH. Evaluating the performance of HIV testing in infants exposed to antiretroviral prophylaxis within South Africa's prevention of mother to child transmission programme. PhD thesis. Pretoria: University of Pretoria, 2018. [ Links ]
 Correspondence:
Correspondence:
S Abushkiwa
abushkiwa1985@gmail.com
Accepted 7 December 2020














