Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Child Health
versión On-line ISSN 1999-7671
versión impresa ISSN 1994-3032
S. Afr. j. child health vol.15 no.3 Pretoria sep. 2021
http://dx.doi.org/10.7196/SAJCH.2021.v15.i3.1772
RESEARCH
The earliest time for haematocrit check after packed red blood cell transfusion among children with anaemia
Q A AdeleyeI; O OniyangiII; L I AuduIII
IMB BS, FMC (Paed); Department of Paediatrics, National Hospital, Abuja, Nigeria
IIMB BS, FWACP (Paed); Department of Paediatrics, National Hospital, Abuja, Nigeria
IIIMB BS, FMC (Paed) Department of Paediatrics, Barau Dikko Teaching Hospital, Kaduna State University, Nigeria
ABSTRACT
BACKGROUND. Haematocrit check after red blood cell transfusion remains relevant in paediatric healthcare. The common practice of checking haematocrit at least 24 hours after transfusion has been challenged by recent evidence supporting much earlier timing. Available data are, however, limited and inconclusive.
OBJECTIVE. To document the changes in haematocrit levels and to determine the earliest time of haematocrit stabilisation after packed red blood cell transfusion in children aged 29 days to 15 years.
METHODS. This was a prospective observational study among 114 eligible participants. Capillary haematocrit was obtained pre-transfusion and at 1, 6, 12, 24 and 48 hours after transfusion. Post-transfusion haematocrit was considered to have stabilised if the difference in mean serial levels within at least 3 consecutive timed intervals was <1.8%. Target outcome was the earliest time to haematocrit stabilisation. Mean haematocrit at the earliest stabilisation time was compared with the expected level using the Student t-test.
RESULTS. The age range of the 103 subjects analysed was 1.5 months - 14 years with a median of 48 months (interquartile range 12 -96 months). Haematocrit increased significantly from pre-transfusion to 1-hour post-transfusion level and declined subsequently. The maximum difference between measurements at 1, 6 and 12 hours was 0.7%. In disaggregated analysis, it was 0.7%, 0.4%, 0.7% and 1.1% among subjects <1 year, 1 - <5 years, 5 - <10 years and 10 - 14 years old, respectively. The mean observed haematocrit at the first hour was similar to the expected level.
CONCLUSION. Post-transfusion haematocrit stabilised at the first hour. Haematocrit checking as early as 1 hour after packed cell transfusion is recommended in children.
Anaemia affects one-quarter of the world population, and about two-thirds of African children under the age of five years.[1] Transfusion with packed red blood cells (or packed cells) is a life-saving intervention among critically ill children, especially in Africa, where severe anaemia is an important cause of child morbidity and mortality.[2,3] It is global practice to check haematocrit or haemoglobin concentration after transfusion; this informs the decision to either administer more blood or discharge the patient if there is no other reason to remain in hospital. Packed cell transfusion is associated with physiological and biochemical changes in the blood.[4,5] The duration of these changes is crucial to the time that post-transfusion haematocrit begins to stabilise, and therefore the earliest time it can be reliably checked.[4]
Since the advent of blood transfusion, the optimal time for performing post-transfusion haematocrit check has not been conclusively determined. Contrary to the common, but largely unsubstantiated, practice of checking it at least 24 hours after transfusion,[4] recent data have supported much earlier timing.[6] In Israel, Glatstein et al.,[6] in a prospective study of 24 preterm neonates compared 15-minute and 6-hour haematocrit levels after transfusion. They found haematocrit stabilisation from the 15th minute. In a similar study of 20 neonates in the USA, Sekhsaria and Fomufod[7] showed that haematocrit levels stabilised from the first hour as compared with the 6-hour level. Audu et al.,[81 at National Hospital, Abuja, Nigeria, compared haematocrit levels at 1, 6, 12, 24 and 48 hours among 40 term and preterm infants aged 0 to 3 months old. Even though levels at 1 and 6 hours were similar in the study, haematocrit stabilisation did not occur until the 12th hour. In a large retrospective review of 564 children aged 1 day to 17 years old in the UK, Davies et al.[9]demonstrated similarity between 1- and 7-hour post-transfusion haemoglobin concentration. Among adult subjects, Elizalde et al.[10]in Spain and Wiesen et al.[11]in the USA in 2 separate prospective studies, also reported early haematocrit and haemoglobin stabilisation from the 15th minute.
In many parts of Nigeria, the time of post-transfusion haematocrit check varies from 6 to 72 hours, with little or no supporting evidence[12,13] No doubt, early timing ofpost-transfusion haematocrit will obviate unnecessary delay in clinical decision-making. Relevant studies on timing post-transfusion haematocrit checks are still limited, especially in Africa, and available data show inconclusive results. Also, prospective studies on post-transfusion haematocrit equilibration have only targeted young infants and adults. Hence, the present study was conducted in children above the neonatal age group up to 15 years old. We hoped the result would augment the pool of evidence-based data on the optimal timing of posttransfusion haematocrit in children.
We aimed to document the changes in haematocrit after packed cell transfusion and to determine the earliest time that post-transfusion haematocrit stabilised. It was hypothesised that the mean haematocrit at 1 hour was not significantly different from levels at 6, 12, 24 and 48 hours after packed cell transfusion of post-neonatal children.
transfusion haematocrit stabilised. It was hypothesised that the mean haematocrit at 1 hour was not significantly different from levels at 6, 12, 24 and 48 hours after packed cell transfusion of post-neonatal children.
Methods
Setting
National Hospital is a tertiary referral centre, located in Abuja -Nigeria's Federal Capital Territory (FCT). The FCT has a population of 1.4 million people (2006 census) and an annual growth rate of 9.3%.[141 The emergency and inpatient paediatric units with a 47-bed capacity receive post-neonatal children up to the age of15 years from primary, secondary and occasionally other tertiary centres within the FCT and neighbouring states. Among the leading reasons for hospitalisation are malaria, acute respiratory infection, diarrhoeal disease and sickle cell disorders.[15]
Design
This was a prospective observational study of anaemic children aged between 29 days and 15 years old admitted to the paediatric department of National Hospital, Abuja (NHA).
Sample size estimation
The sample size was calculated using the formula:[16]
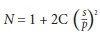
A minimum sample size of 110 was obtained to detect a change of 2.7%[17] in post-transfusion haematocrit levels with a power of 90% and 5% level of significance.
Ethical considerations
Ethical approval was obtained from the Ethics Committee of the NHA before embarking on the study (NHA/EC/053/2016). The parent or guardian consented and the child assented (if applicable) before recruitment. The subjects' morbidity was managed according to the unit's protocol.
Recruitment and procedure
The study was conducted between 1 May 2017 and 6 March 2018. Patients who required packed cell transfusion were recruited consecutively. Those with evidence of active haemolysis based on the presence of haemoglobinuria, elevated urine urobilinogen, or reticulocytosis were excluded from the study. Other exclusion criteria were active bleeding, transfusion within the previous 48 hours, and progressive decline in serial post-transfusion haematocrit to pre-transfusion level or less. Sociodemographic data were obtained and relevant anthropometric measurements were taken. The pulse and respiratory rates were counted and the blood pressure was measured. Results of reticulocyte count (as part of complete blood count) and haemoglobin phenotype were obtained from the participants' index admission records.
Blood transfusion
Preparation of packed cells was done in the blood bank by medical laboratory scientists and stored in citrate-phosphate-dextrose solution with adenine (CPDA). The required volume for transfusion was calculated based on the unit policy using the formulae:

Subjects with haemodynamic instability as determined by easy fatiguability, tachypnoea, tachycardia and tender hepatomegaly had 1 mg/kg of intravenous furosemide before transfusion. Transfusion was administered over approximately 4 hours.
Blood sampling and haematocrit measurement
A capillary blood sample was obtained just before transfusion commenced. Samples were also taken at 1, 6, 12, 24 and 48 hours after completion of transfusion. Blood samples were obtained from the medial or lateral plantar surface of the heel in subjects <6 months old, and from the lateral palmar surface of the middle or ring finger in those >6 months old.[20] A sterile puncture (not more than 2 mm deep) was made with a lancet, 3 - 5 minutes after application of a topical anaesthetic agent (10% Xylocaine pump spray). Blood was allowed to flow as freely as possible into a heparinised capillary tube until three-quarters full and was sealed with plasticine.'[20,21] Given a volume of 70 μL per capillary tube, 0.05 mL of blood was obtained from each subject before transfusion and a total of 0.26 mL was obtained after completion of transfusion. A donor blood sample was obtained from the blood bag into a heparinised capillary tube at the end of transfusion.
The samples were spun with a micro-haematocrit centrifuge at 10 000 rpm (Heraeus, Germany) for 5 minutes. The haematocrit levels were then obtained on a micro-haematocrit reader.
Statistical analysis
Analysis was done with the Statistical Package for Social Sciences (IBM Corp., USA), version 25. The data were disaggregated into <1 year, 1 - <5 years, 5 - <10 years and 10 - 14 years. The mean and standard deviation (SD) were computed for pre-transfusion haematocrit and haematocrit levels at 1, 6, 12, 24 and 48 hours after transfusion. The differences between the mean post-transfusion haematocrit at 1 hour and other timed intervals were calculated. A clinically significant difference was taken to be more than 1.8%.[10·11,17] The student i-test was used to compare the mean pre-transfusion haematocrit and each of the serial post-transfusion levels. The same statistic was also used to compare the mean haematocrit at the earliest time of stabilisation and the mean expected haematocrit. Statistically significant difference was set at p <0.05.
Definitions
Post-transfusion haematocrit stabilisation
Stabilisation was considered to have occurred from the time beyond which there was no clinically significant difference in mean haematocrit within at least 3 consecutive timed intervals. A difference in post-transfusion haematocrit levels that was <1.8% was used as the basis for stabilisation, as changes in haemoglobin in the range of 6.6 - 10 g/L (haematocrit 1.98 - 3%) are considered clinically significant.'171
Expected post-transfusion haematocrit
This was calculated from the primary formulae used in estimating the required volume of packed cells:[18]
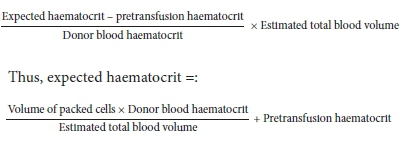
The total blood volume was estimated using 80 mL/kg for subjects <12 months old and 75 mL/kg for those >12 months old.[19]
Results
A total of 114 potential participants were recruited, of whom 11 were excluded from analysis. Two sickle cell anaemia subjects had a rapid decline in serial post-transfusion haematocrit levels that necessitated repeat transfusion within 24 hours. In 8 subjects, data on serial post-transfusion haematocrit were not complete. In 1 subject, the reticulocyte count was elevated. The age range of the 103 participants analysed was 1.5 months - 14 years (median 48 months) (interquartile range 12 - 96 months). Table 1 shows the age-group distribution of subjects, with a preponderance of 1 - <5 and 5 - <10 years age groups. Male subjects predominated in each group, and overall they were significantly more, with a male:female ratio of 1.7:1 (p <0.023). Table 2 shows the vital signs of subjects prior to blood transfusion.
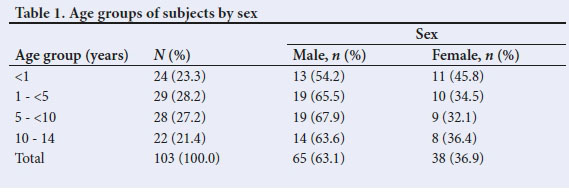
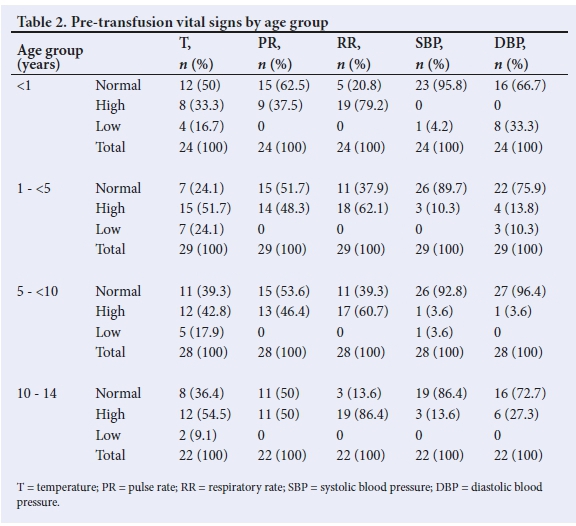
Table 3 displays the changes in haematocrit from pre-transfusion level to serial posttransfusion measurements. Haematocrit increased significantly 1 hour after transfusion (p<0.001). Within each age group, all timed post-transfusion levels were higher than the pre-transfusion level (p<0.001). Haematocrit progressively declined from 1-hour posttransfusion level through the 48-hour assessment. There were two exceptions: the first was in the <1-year group where the haematocrit did not change between 12 and 24 hours, and the second was in the 5 - <10-year group where the haematocrit was the same between 24 and 48 hours.
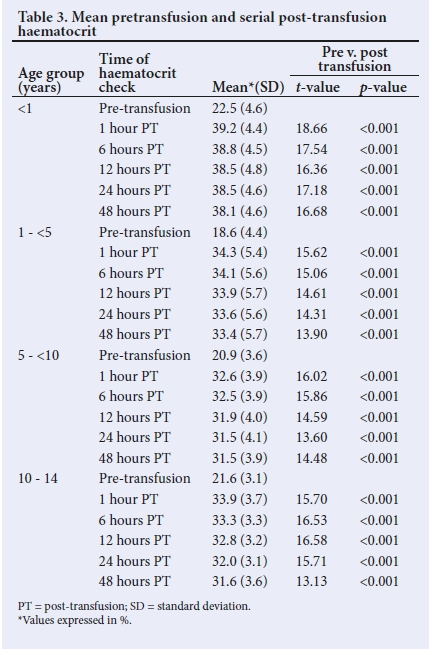
In aggregated analysis, the differences between the 1st-hour haematocrit and subsequent measurements were all less than 1.8%, as shown in Table 4.
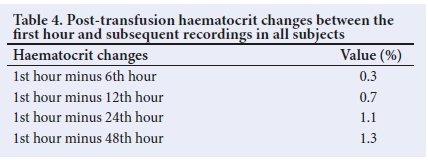
Table 5 shows the differences when the data were disaggregated. The differences between the 1st-hour assessment and other assessments were mostly less than 1.8% in all age groups; the exceptions were in subjects 10 - 14 years old in whom differences of more than 1.8% were observed with the 24th- and 48th-hour levels.
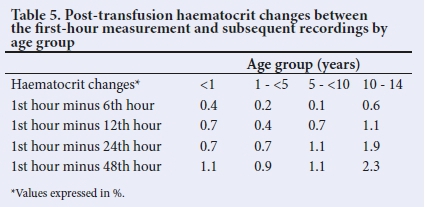
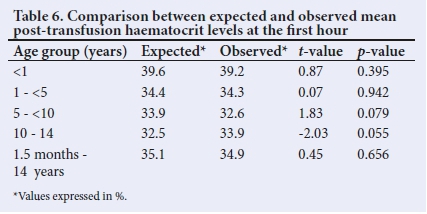
Paired student t-test comparison showed that the expected haematocrit was similar to the observed haematocrit, 1 hour after transfusion (Table 6). This was seen in both aggregated and disaggregated analysis.
Discussion
The present study demonstrated that haematocrit stabilised at the first hour after packed cell transfusion in children aged 1.5 months - 14 years old. It agrees with previous studies that have shown that posttransfusion haematocrit stabilises much earlier than 24 hours. In specific terms, our study supports the 1-hour stabilisation timing reported by Davies et al.,[9] who examined subjects of comparable age in the UK. The similarity between the two studies underpins early post-transfusion haematocrit checks in general and 1st-hour checks in particular. Similarly, Sekhsaria and Fomufod[7] in their study of neonates, reported 1-hour stabilisation timing of posttransfusion haematocrit checks. This may indicate that the physiological differences between neonates and older children do not influence the time of haematocrit equilibration after packed cell transfusion. We did not obtain samples earlier than 1 hour for comparison. It is plausible that haematocrit may have stabilised earlier than 1 hour, as reported in similar studies among preterm and adult populations[6,10,11]
Stabilisation of haematocrit in the present study occurred earlier than the 12 hours reported from the same centre by Audu et al.,[8] among young infants up to the age of 3 months. Meanwhile, the methodology in these two studies was similar in being prospective, in performing 5-point serial post-transfusion checks and in using at least 3 consecutive levels as the basis for stabilisation. The larger sample size in this study and the difference in subject characteristics could, however, account for the variation in outcome. Nonetheless, the findings in both studies support early stabilisation of post-transfusion haematocrit in children.
The outcome of the present study was the same in both aggregated and disaggregated analysis, which indicates that, among postneonatal children, age does not influence the time of haematocrit equilibration after packed cell transfusion. It also suggests that stabilisation of post-transfusion haematocrit may not be affected by physiological changes that occur with age in children, such as decreasing extracellular volume and increasing haematocrit. Lack of age influence was also demonstrated in similar studies, though in different age groups - preterm neonates, by Glatstein et al.,[6] and adults, by Elizalde et al.,[10] and Wiesen et al.[11] Among participants who were 10 - 14 years old in this study, it was not exactly clear why haematocrit levels at 24 and 48 hours after transfusion differed from the 1st-hour level by more than 1.8%. Nonetheless, it may suggest that while haematocrit stabilises at the 1st hour after transfusion, its readjustment after equilibration begins at 24 hours in this group of children. A similar study in the future involving a large sample size of children in this category may provide more information in this area.
Expected haematocrit was calculated using the primary formula that has been shown as most accurately predictive of the target post-transfusion haematocrit.[18] The present study demonstrated an agreement between the expected haematocrit and 1-hour levels in each of the age sub-groups and when all participants were analysed together. This finding further supports the argument that haematocrit stabilisation occurred from this time, irrespective of age. Audu et al.[8]also demonstrated the same to corroborate the stabilisation of post-transfusion haematocrit at 12 hours.
The steady decline in serial post-transfusion haematocrit observed in this study is similar to what was reported by Davies et al.[9] among children aged 1 day - 17 years. While the actual reason for this decline may be difficult to propose, the haemodilution following efflux of red-cell sodium into the intravascular compartment after transfusion may be partly responsible.[221 Meanwhile, the plasma volume and red cell concentration of sodium have been reported to increase after red cell transfusion, and then normalise in 24 hours.[4,22] However, the outcome of our study suggests that such physiological and biochemical changes, or a possible covert loss of red blood cells, do not result in clinically significant changes in haematocrit levels from the first hour after packed cell transfusion.
The findings of the present study indicate that haematocrit checks can be reliably done at the first hour after packed cell transfusion. Adoption of this timing would minimise unnecessary delay in repeating transfusion, or in discharging a patient from the hospital. This is relevant, especially in resource-poor settings where bedspace and healthcare facilities are limited. Although smaller sample sizes in the disaggregated analysis could have influenced the validity of stabilisation time in each age group, the results were not different from that of the composite analysis.
Conclusion
This study demonstrated that post-transfusion haematocrit stabilised at the first hour after packed cell transfusion in children aged 1.5 months - 14 years. We therefore recommend checking post-transfusion haematocrit as early as the first hour in children aged 1.5 months - 14 years. A future similar study that examines haematocrit stabilisation earlier than 1 hour, and one that involves a large number of children aged 10 - 14 years, will be beneficial.
Declaration. The authors declare the originality of this paper. Where other persons' ideas were mentioned, they have been referenced appropriately.
Acknowledgements. We acknowledge the diligent efforts of Dr Aba Moses and Dr Aikoriogie Osaheni who assisted with blood sample collection. Our profound appreciation goes to all staff in emergency and inpatient paediatric units of the hospital who were involved in the clinical management of our participants. We are also grateful to all the children and their parents who participated in the study.
Author contributions. All authors took part in the development of the research concept and design. QAA co-ordinated the collection of data. QAA and LIA analysed the data and drafted the manuscript. QAA and OO did the literature search. LIA and OO edited the manuscript. All authors approved the final manuscript.
Funding. The cost of blood transfusion was borne by the parents and guardians as part of the cost of clinical care. The micro-haematocrit centrifuge used for the study was the property of the Department of Paediatrics. Other sundry expenses were borne by the authors.
Conflicts of interest. None.
References
1. McLean E, Cogswell M, Egli I, Wojdyla D, de Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993 - 2005. Public Health Nutr 2009;12(4):444-454. https://doi.org/10.1017/s1368980008002401 [ Links ]
2. Dhingra DN. Blood safety in the developing countries and WHO initiatives. Vox Sanguinis 2002;83:173-177. https://doi.org/10.1111%2Fj.1423-0410.2002.tb05295.x [ Links ]
3. Lackritz EM, Campbell CC, Ruebush TK, Hightower AW, Wakube W, Were JB. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet 1992;340(8818):524-528. https://doi.org/10.1016/0140-6736(92)91719-o [ Links ]
4. Ness PM, Rothko K. Principles of red blood cell transfusion. In: Hoffman R, editors. Hematology: Basic Principles and Practice. 6th ed. New York: Churchill Livingstone, 2013:1642-1652. [ Links ]
5. Gabrio BW, Stevens AR, Finch CA. Erythrocyte preservation. III. The reversibility of the storage lesion. J Clin Invest 1954;33(2):252-256. https://doi.org/10.1172%2Fjci102893 [ Links ]
6. Glatstein M, Oron T, Barak M, Mimouni FB, Dollberg S. Posttransfusion equilibration of hematocrit in hemodynamically stable neonates. Pediatr Crit Care Med 2005;6(6):707-708. https://doi.org/10.1097%2F01.pcc.0000185490.19677.b4 [ Links ]
7. Sekhsaria S, Fomufod A. Readjustment of hematocrit values after packed red cell transfusion in the neonate. J Perinatol 1991;11(2):161-163. http://europepmc.org/abstract/med/1890477 [ Links ]
8. Audu LI, Otuneye AT, Mairami AB, Mshelia LJ, Nwatah VE. Posttransfusion haematocrit equilibration: Timing posttransfusion haematocrit check in neonates at the National Hospital, Abuja, Nigeria. Int J Pediatr 2015;2015:1-5. https://doi.org/10.1155%2F2015%2F175867 [ Links ]
9. Davies P, Robertson S, Hegde S, Greenwood R, Massey E. Calculating the required transfusion volume in children. Transfusion 2007;47(2):212-216. https://doi.org/10.1111%2Fj.1537-2995.2007.01091.x [ Links ]
10. Elizalde JI, Clemente J, Marin JL, et al. Early changes in hemoglobin and hematocrit levels after packed red cell transfusion in patients with acute anemia. Transfusion 1997;37(6):573-576. https://doi.org/10.1046%2Fj.1537-2995.1997.37697335150.x [ Links ]
11. Wiesen AR, Hospenthal DR, Byrd JC, Glass KL, Howard RS, Diehl LF. Equilibration of hemoglobin concentration after transfusion in medical inpatients not actively bleeding. Ann Intern Med 1994;121(4):278-280. https://doi.org/10.7326%2F0003-4819-121-4-199408150-00009 [ Links ]
12. Adedoyin OT, Afolabi JK, Oyeyemi B. Proposed formulae for determining blood transfusion requirements in children with severe anaemia. Niger J Paed 2005;31(1):25-28. https://doi.org/10.4314%2Fnjp.v31i1.12084 [ Links ]
13. Oniyangi O, Ahmed P, Otuneye O. Strokes in children with sickle cell disease at the National Hospital Abuja Nigeria. Niger J Paed 2013;40(2):158-164. https://doi.org/10.4314/njp.v40i2,10 [ Links ]
14. Report of Nigeria's National Population Commission on the 2006 Census. Population Dev Rev 2007;33(1):206-210. https://www.jstor.org/stable/25434601 [ Links ]
15. Ahmed P, Babaniyi I, Yusuf K, et al. Vitamin D status and hospitalisation for childhood acute lower respiratory tract infections in Nigeria. Paed Int Child Health 2014;35(2):151-156. https://doi.org/10.1179%2F2046905514y.0000000148 [ Links ]
16. Dell RB, Holleran S, Ramakrishnan R. Sample size determination. ILAR J 2002;43(4):207-213. https://doi.org/10.1093 https://doi.org/10.1093 [ Links ]
17. Wintrobe MM. Clinical Hematology. 8th ed. Philadelphia: Lea and Febiger, 1981:8. [ Links ]
18. Morris KP, Naqvi N, Davies P, Smith M, Lee PW. A new formula for blood transfusion volume in the critically ill. Arch Dis Child 2005;90(7):724-728. https://doi.org/10.1136%2Fadc.2004.062174 [ Links ]
19. Butterworth JF, Mackey DC, Wasnick JD. Morgan & Mikhail's Clinical Anaesthesiology. 5th ed. New York: McGraw Hill Education, 2013:893. [ Links ]
20. Briggs C, Bain BJ. Basic Haematological Techniques. In: Bain BJ, Bates I, Laffan MA, Lewis SM, editors. Dacie and Lewis Practical Haematology. 11th ed. London: Elsevier Churchill Livingstone, 2011:23-56. [ Links ]
21. World Health Organization. WHO guidelines on drawing blood: Best practices in phlebotomy. 2010. Geneva: WHO. https://www.who.int/infection-prevention/publications/drawing_blood_best/en/ (accessed 6 December 2016). [ Links ]
22. Maizels M, Paterson J. Survival of stored blood after transfusion. Lancet 1940; 236(6110):417-420. https://doi.org/10.1016%2Fs0140-6736%2800%2998520-9 [ Links ]
 Correspondence:
Correspondence:
Q A Adeleye
qadri_adeleye@yahoo.com
Accepted 6 January 2021














