Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.15 no.1 Pretoria Abr. 2021
http://dx.doi.org/10.7196/sajch.2021.v15i1.1756
RESEARCH
Exploring determinants of under-5 stunting in Malawi using a generalised linear mixed model
R F AfolabiI, II; M F PalamuleniII
IPhD; Department of Epidemiology and Medical Statistics, Faculty of Public Health, College of Medicine, University of Ibadan, Nigeria
IIPhD; Population Studies and Demography Programme and Population and Health Research Entity, Faculty of Humanities, North-West University Mmabatho, South Africa
ABSTRACT
BACKGROUND: Although several studies have examined determinants of stunting, most fail to account for unobserved heterogeneity in clustered survey data
OBJECTIVE: To investigate the determinants of stunting among Malawian children under-5 years of age
METHODS: The study utilised cross-sectional data on 5 686 children aged 0 - 59 months extracted from the 2015 - 2016 Malawi Demographic and Health Survey dataset. Data were analysed using a generalised linear mixed model applied to clustered data
RESULTS: Children who were female (adjusted odds ratio (aOR) 0.83; 95% confidence interval (CI) 0.73 - 0.94), from wealthier households (aOR 0.68; CI 0.58 - 0.81), and whose mothers were overweight/obese (aOR 0.78; CI 0.65 - 0.94), aged 25 - 34 years (aOR 0.76; CI 0.62 - 0.93) or had at least a secondary level education (aOR 0.73; CI 0.56 - 0.94) were less likely to be stunted. The likelihood of stunting was higher among children who were anaemic (aOR 1.38; CI 1.20 - 1.59) and in whom respondents reported small birth size (aOR 1.61; CI 1.34 - 1.93). Age >12 months, being a twin or triplet and living in the Central region of Malawi also increased a child's risk of being stunted. About 3.0% of the variance in likelihood of being stunted occurred across communities (clusters
CONCLUSIONS: Hidden community variations of child stunting in clustered-survey data need to be accounted for. Stunting strategies should be context specific
Stunting is a risk factor for poor health and psychosocial development arising from in utero and/or early childhood malnutrition.[1] As the most prevalent form of child malnutrition, stunting remains a public health problem.[2] Although efforts have been put in place by various stakeholders to ameliorate these negative impacts through several nutrition interventions,[1,3,4] reduction in the trend of malnutrition in under-5-year-olds (hereafter 'under-5 malnutrition') in Malawi and many sub-Saharan African countries is still suboptimal. Worryingly the 2015 - 2016 Malawi Demographic Health Survey (MDHS) reported a prevalence of 37.1% stunting in under-5s.[5]
Previous studies have used varying statistical approaches to examine factors associated with stunting in Malawi,[6-9] in other sub-Saharan African countries[10-13] and elsewhere.[14-16] These studies attest to individual setting or country peculiarities and the multifaceted nature of the associated risk factors. In Malawi, the likes of quantile regression,[6] linear random effect model,[17] generalised estimating equation,[9] logistic regression,[7,18] and multilevel logistic regression model[8] have been employed to identify determinants of stunting. Many of these studies, however do not account for unobserved heterogeneity in clustered-survey data.
We sought to identify potential risk factors of stunting among under-5s in Malawi using a generalised linear mixed model (GLMM) approach. The GLMM was employed to appropriately adjust for the peculiar nature of hierarchical data, including missing observation, using the most recent MDHS clustered dataset. This was necessary to attain objective and unbiased inferences on predictors of child stunting.
Methods
Study design, setting and population
The present analysis used the 2015-16 MDHS data, a cross-sectional design aimed at providing population and maternal-and-child-health indicators.[5] Ethical approval for the parent study was obtained from the National Health Sciences Research Committee, Malawi. The Demographic and Health Surveys Program approved the use of the dataset for the present analysis.
Malawi has a population of 18.1 million, of whom 2.9 million were under 5 years of age in 2016. Only 16% of the population resided in urban areas.[4,5] The multistage cluster sampling technique was used for the survey, based on the sampling frame containing enumeration areas (EAs), adopted from the 2008 Malawi Population and Housing Census. At the first stage, 850 EAs referred to as clusters (communities) were selected as the primary sampling units. A total of 26 361 of the sampled households within the selected EAs were interviewed at the second stage. The detailed sampling procedure has been reported previously.[5]
Of 6 033 under-5s eligible for anthropometric measurement within households, 5 686 (94%) had complete and valid height measurements.[5] The term cluster' is used interchangeably with community' to indicate children living in the same household within the same geographical location in this study
Study variables
The outcome of interest is the stunting of under-5s measured by height-for-age. With the use of a ShorrBoard measuring board (Shorr Productions, USA), length of children aged <24 months was measured supine and those aged >24 months were measured while standing. The height-for-age variable was transformed into a binary variable (stunting present or absent) depending on whether a child's z-score was below or above -2 standard deviations (SDs) from the median of the World Health Organization (WHO) reference population.[5] The independent variables included to define child, maternal and household characteristics are presented in Appendix 1 (http://www.sajch.org.za/public/files/1756.pdf).
Statistical methods of analysis
Descriptive statistics and GLMM with a binomial random distribution and logit link function were used for the analysis. Besides the use of GLMM at a bivariate level to identify individual explanatory variables' influence on child stunting, GLMM was further used to identify determinants of stunting among under-5s using a 4-stage approach at multivariate level. Models 1, 2 and 3 respectively included variables to define child, maternal and household characteristics, irrespective of their significance status at the bivariate level. Thereafter, significant predictors (having 95% confidence intervals (CIs) not including 1) from models 1-3 were included in final model 4. The odds ratios (OR) and their CIs are reported. The intra-community correlation coefficient (ICC) that measures the proportion of variance explained by clustering in hierarchical data is reported. An ICC >2% implies existence of a significant cluster level effect which calls for a multilevel approach.[19,20] Akaike information criteria (AIC) values are also reported for model comparison; the model with the lowest value was adjudged as being more adequate.[21,22] All analyses were carried out at 5% level of significance, using STATA 14 SE (StataCorp., USA).
Model description
The GLMM is an extension of generalised linear models which takes into consideration all contextual (level-two) information.[23] The model specifically combines and estimates both the fixed- and random-effects appropriate for clustered data. The model could be briefly described as follows.
Let Yijbe the stunting status of ilh under-5 in jth cluster defined as

the GLMM can be described as follows.
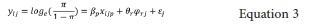
where
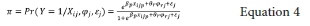
Level-one p-variable is represented by xijp (varies within and between clusters); level-two r-variable is represented by φrj (varies only between clusters); and the random intercept is ej.
The four-stage random intercept model approach adopted was subject to the grouping of explanatory variables into child, maternal and household characteristics. In model 1, simultaneous effects of child's age, sex, birth size, birth type, birth interval, anaemia status and recent illness on stunting were estimated. While model 2 includes mother's age, education, body mass index (BMI), marital status, employment, number of living children and breastfeeding status, model 3 simultaneously estimates the effect of regions, residence, wealth quintile, household-head sex, source of drinking water and type of toilet facility on stunting. Lastly, model 4 estimates the combined effects of the significant child, maternal and household characteristics on stunting. In each of the four models, ICC was computed using the estimated random intercept variance.
Results
Background characteristics of children under 5
Children and mother's mean (SD) ages were 30.0 (17.1) months and 26.0 (6.7) years, respectively. Household and demographic characteristics categorised by stunting status are described in Table 1. Most children were aged >24 months (61.8%) and lived in rural settings (87.4%). Only 1% of respondents were covered by health insurance and one-fifth had attained secondary or higher education. The proportion of stunting decreased with increasing maternal education and BMI.
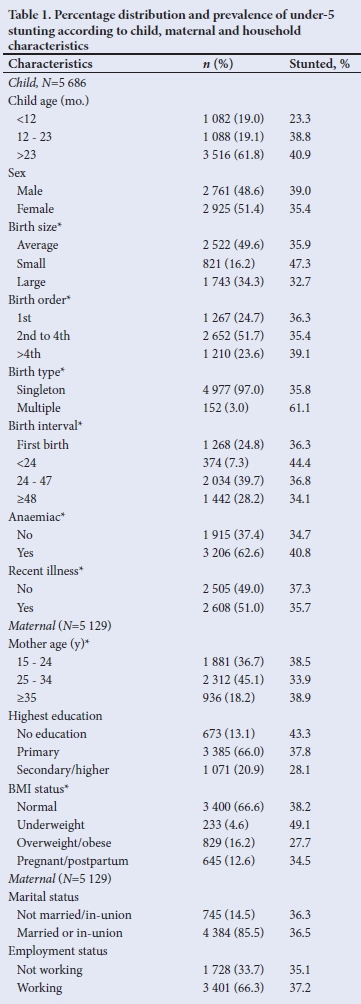
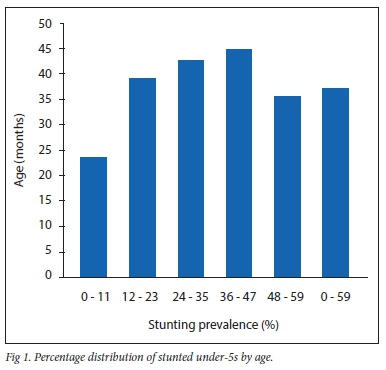
Pattern of stunting by child age
Mean (SD) ages of stunted and non-stunted children were 31.7 (15.6) and 29.0 (17.8) months, respectively. The prevalence of stunting increased till age 12 - 23 months, higher than 37.1% overall prevalence and remained relatively static during the third and fourth years of life, with a slight decline thereafter (Fig. 1).
Factors influencing stunting among under-5s
The crude and adjusted associations of stunting with child, maternal, household and combined significant characteristics are set out as models 1 - 4 in Table 2. All selected child-related variables, except birth order and recent illness, were significantly associated with stunting. Among the maternal characteristics, only mother's age, education and BMI individually impacted on stunting. Except for household-head sex and toilet type, all household characteristics were significantly associated with stunting.
Model 4 (with the lowest AIC (5 696)) had the best fit. In model 4, the likelihood of being stunted was higher among children aged >23 months (aOR 2.95; CI 2.16 - 4.02), in children who were anaemic (aOR 1.38; CI 1.20 - 1.59), twins or triplets (aOR 2.47; CI 1.66 - 3.68) and had small birth size (aOR 1.61; CI 1.34 - 1.93). It was reduced among females (aOR 0.83; CI 0.73 - 0.94). Stunting was decreased in children of mothers aged 25 - 34 years (aOR 0.76; CI 0.62 - 0.93), who were overweight or obese (aOR 0.78; CI 0.65 - 0.94) and had secondary or higher education (aOR 0.73; CI 0.56 - 0.94). While children from Central Malawi (aOR 1.26; CI 1.03 - 1.55) were more likely to be stunted, those from wealthier households (aOR 0.68; CI 0.58 - 0.81) were less likely to be stunted. The ICC was >2% in all the models, suggesting the appropriateness of the choice of our model. A relatively small but significant (ICC 3.0%; p<0.05) variation in under-5 stunting was attributed to clustering effect at community level (model 4, Table 2).
Discussion
We employed GLMM to identify risk factors of stunting among under-5s in Malawi, using nationally representative data. Saliently, the models collectively demonstrate holistic predictors of stunting which include childs age, sex, birth size, birth type, anaemia status, maternal age, education, BMI, the region of residence and household wealth. We found a significant variation in child stunting owing to clustering effect at community level. Notwithstanding many studies on childhood stunting in Malawi, our study was the first to have accounted for the problem of dependency structure and unexplained variation in clustered-survey data using GLMM. This is important as child stunting may be inappropriately examined if the nested nature of the dataset like MDHS is ignored.[23]
The observed significant variation between communities is expected as under-5s belonging to the same community of households usually share common unobserved heterogeneity[22] This suggests that some communities may be at high risk of stunting with others at low risk. The implication is that some determinants of child stunting in this study, which may be propelled by environmental, cultural behavioural and biological factors, remain unmeasured. This may need further attention as we confirmed the prevalence of under-5 stunting in Malawi to be very high.[2] The prevalence is higher compared with some neighbouring countries such as Zimbabwe (29.2%) and Tanzania (35.5%).[13,24]
Increasing age was significantly associated with stunting. Studies in Malawi[6,8] and other sub-Saharan African countries have also reported similar findings.[11,13,21,25-28] The finding of the lowest prevalence within the first 6 months may likely be linked to the protective influence of breastfeeding, with nearly two-thirds of infants reported to be exclusively breastfed in Malawi.[5] Child's sex was another predictor of stunting in concordance with other studies.[9,20,24,29,30]
Female children were less prone to be stunted. This sex disparity may be associated with contextual behavioural patterns of the caregivers, including females' preferential care.[24] However, other studies[13,31,32] found females were more stunted. The racial disparities across regions and countries might account for this difference.
Infants with respondent-reported small birth size were more stunted. Other literature[9,20,24,27] has corroborated this finding. As newborn size reflects the intrauterine condition, in addition to preterm birth, small birth size may be partly influenced by maternal nutrition and health status during pregnancy.[31,33] Maternal prenatal healthcare and nutritional well-being, therefore, need attention, especially in preventing low birthweight. The finding that being a twin/triplet heightened the risk of being stunted aligns with other literature.[9,34] This could likely be as a result of maternal food insecurity or inappropriate child feeding practice;[5] for instance, twins tend to struggle to compete for breast milk and other food supplements at the same time, especially in a poor economy setting.[28]
Anaemic children were more prone to be stunted, in tandem with other findings.[12,26,28,35] One study[36] describes a significant relationship between infection, anaemia and stunting. For instance, a study[37] in Malawi has documented that child anaemia could be a product of malaria-in-pregnancy or low birthweight. Relatively few under-5s had malaria-related anaemia according to the MDHS report.[5] Enhancing iron supplementation and antimalarial control in children is a crucial public health intervention.[3,37]
Mothers educational attainment positively impacts on the quality of healthcare a woman receives before, during and after pregnancy or offers her child after delivery.[38] We found a strong association between higher maternal education and lower child stunting. This aligns with earlier findings in the literature.[20,25-27] Addressing stunting requires empowerment of women through education.[20] Evidence of a strong positive association between higher educational attainment and wealth status is replete in the literature.[25-27,32] Lower household wealth status was a significant predictor of stunting in this study, as previously described.[20,25-27] Nearly half of the children resided in poor households, a pointer to inability of the household to procure needed food or medical care.[30] Maternal BMI is indicative of maternal nutritional well-being.[33] The present study suggests that maternal overweight or obesity is linked to decreased child stunting; this has been corroborated by a study in Ethiopia.[39] In contrast, a Kenyan study reported a higher tendency to being stunted with maternal overweight.[25]
Child stunting remains a critical child health problem in the Central region which constitutes 42% of the total population. Risks of stunting were 26% and 14% higher in the Central region compared with under-5s living in northern and southern regions, respectively. These regional variations in stunting can partially be associated with regional differences in geography, race, culture or belief.[18] While one previous study confirms,[30] another found no significant regional differences.[9] The contrasting result may also be attributed to the use of an older MDHS dataset or a statistical method which impedes likelihood-based inference.[23]
Study limitations and strengths
The present study has some limitations. First, the study design is cross-sectional; the analysed variables can only provide evidence of a statistical relationship but not a causal relationship between the variables and under-5 stunting status. Second, there is a possibility of recall bias as the study included self-reported data without any means of verification. Also, the use of secondary data restricted our potential to sufficiently assess the influence of some characteristics such as dietary intake, feeding practices and national income as drivers of child stunting. Nonetheless, the study has been strengthened by the use of a large nationally representative dataset. Besides, the strength of the work includes carefully adjusting for the potential confounders of stunting, thus taking context into consideration. Also, we uncovered and quantified the extent of hidden characteristics from the effects of the observed characteristics, and this enhanced reliable inferences.
Conclusions
Our fin dings emphasise the need to account for hidden characteristics in clustered-survey data. In Malawi, sustained and broadened efforts must be intensified to achieve a 40% reduction in stunting as enshrined in the WHO Global Targets for 2025.[1,31] The relatively small but important observed unexplained variations in stunting across communities should inform intervention strategies that sufficiently address context.
Declaration. None.
Acknowledgements. The authors acknowledge the DHS Program for granting free access to the data used for the study.
Author contributions. RFA conceived and designed the study. RFA analysed the data while RFA and MEP interpreted the analysed data. RFA drafted the original manuscript. RFA and MEP reviewed and edited the manuscript. Both authors have read and agreed to the published version of the manuscript.
Funding. None.
Conflicts of interest. None.
References
1. UNICEF, WHO, and World Bank group. Levels and trends in child malnutrition: Key findings of the 2019 Edition of the Joint Child Malnutrition Estimates. Geneva, 2019. https://data.unicef.org/wp-content/uploads/2019/04/Joint-malnutrition-estimates-March2019-1.pdf (accessed 10 December 2019). [ Links ]
2. De Onis M, Borghi E, Arimond M, et al. Prevalence thresholds for wasting, overweight and stunting in children under 5 years. Public Health Nutr 2019;22(1):175-179. https://doi.org/10.1017%2Fs136898001800243 [ Links ]
3. BhuttaZA, Das JK, Rizvi A, et al. Evidence-based interventions for improvement of maternal and child nutrition: What can be done and at what cost? Lancet 2013;382(9890):452-477. https://doi.org/10.1016%2Fs0140-6736%2813%29 [ Links ]
4. United States Agency for International Development (USAID). Malawi: Nutrition Profile. 2018. https://www.usaid.gov/global-health/health-areas/nutrition/countries/malawi-nutrition-profile (accessed 10 December 2019). [ Links ]
5. National Statistical Office (NSO), Information and Communication Technology (ICT). Malawi Demographic and Health Survey 2015-16. Zomba, Malawi, and Rockville, Maryland, USA: 2017. http://dhsprogram.com/pubs/pdf/FR319/FR319.pdf (accessed 10 April 2019). [ Links ]
6. Mtambo OPL, Masangwi SJ, Kazembe LNM. Analysis of childhood stunting in Malawi using Bayesian structured additive quantile regression model. Int J Stat Appl 2014;4(3):161-171. https://doi.org/10.5923/j.statistics.20140403.04 [ Links ]
7. Osendarp SJM, Shilpi F, Gondwe T, et al. Determinants of reductions in childhood stunting in Malawi's community-based nutrition programs. Health, Nutrition, and Population discussion papers. Washington, DC 20433, USA: 2019. World Bank Group, http://documents.worldbank.org/curated/en/297601565964816621/Determinants-of-Reductions-in-Childhood-Stunting-n-Malawis-Community-based-Nutrition-Programs (accessed 12 April 2019). [ Links ]
8. Chikhungu LC, Madise NJ, Padmadas SS. How important are community characteristics in influencing children's nutritional status? Evidence from Malawi population-based household and community surveys. Health Place 2014;30:187-195. https://doi.org/10.1016%2Fj.healthplace.2014.09.006 [ Links ]
9. Ntenda PAM, Chuang YC. Analysis of individual-level and community-level effects on childhood undernutrition in Malawi. Pediatr Neonatol 2018;59(4):380-389. https://doi.org/10.1016%2Fj.pedneo.2017.11.019 [ Links ]
10. Teferi MB, Hassen HY, Kebede A, et al. Prevalence of stunting and associated factors among children aged 06 - 59 months in Southwest Ethiopia: A cross-sectional study. J Nutr Health Food Sei 2016;4(6):1-6. https://doi.org/10.15226%2Fjnhfs.2016.00180 [ Links ]
11. Garcia Cruz LM, Gonzalez Azpeitia G, Reyes Súarez D, Santana Rodriguez A, Loro Ferrer JF, Serra-Majem L. Factors associated with stunting among children aged 0 to 59 months from the central region of Mozambique. Nutrients 2017;9(5):1-16. https://doi.org/10.3390%2Fnu9050491 [ Links ]
12. Moges B, Feleke A, Meseret S, Doyore F. Magnitude of stunting and associated factors among 6-59 months old children in Hossana Town, Southern Ethiopia. J Clin Res Bioeth2015;6(1):4-11. https://doi.org/10.4172%2F2155-9627.1000207 [ Links ]
13. Maradzika J, Makwara IP, Chipunza S. Factors associated with stunting among children aged 0 to 59 months in Harare City, Zimbabwe. Int J Child Health Nutr 2016;5(1):31-44. https://doi.org/10.6000%2F1929-4247.2016.05.0L5 [ Links ]
14. Manggala AK, Wiswa K, Kenwa M, et al. Risk factors of stunting in children aged 24-59 months. Paediatr Indones 2018;58(5):205-212. https://doi.org/10.14238/pi58.5.2018.205-12 [ Links ]
15. Saha UR, Chattapadhayay A, Richardus JH. Trends, prevalence and determinants of childhood chronic undernutrition in regional divisions of Bangladesh: Evidence from demographic health surveys, 2011 and 2014. PLoS ONE 2020;15(2):e0229677. https://doi.org/10.1371%2Fjournal.pone.0229677 [ Links ]
16. Hossain M, Choudhury N, Abdullah KAB, et al. Evidence-based approaches tc childhood stunting in low and middle income countries: A systematic review. Arch Dis Child 2017;102(10):903-909. https://doi.org/10.1136%2Farchdischild-2016-311050 [ Links ]
17. Sassi M. Evidence of between- and within-household child nutrition inequality in Malawi: Does the gender of the household head matter? Eur J Dev Res 2020;32:28-50. https://doi.org/10.1057/s41287-019-00220-8 [ Links ]
18. Doctor HV, Nkhana-Salimu S. Trends and determinants of child growth indicators in Malawi and implications for the Sustainable Development Goals. AIMS Public Health 20174(6):590-614. https://doi.org/10.3934%2Fpublichealth.2017.6.590 [ Links ]
19. Theall KP, Scribner R, Broyles S, et al. Impact of small group size on neighborhood influences in multilevel models. J Epidemiol Community Health 2011 ;65(8) :688-695. https://doi.org/10.1136/jech.2009.097956 [ Links ]
20. Gebru KF, Haileselassie WM, Temesgen AH, Seid AO, Afework Mulugeta B. Determinants of stunting among under-five children in Ethiopia: A multilevel mixed-effects analysis of 2016 Ethiopian demographic and health survey data. BMC Pediatr 2019;19(176):1-13. https://doi.org/10.1186/s12887-019-1545-0 [ Links ]
21. Takele K, Zewotir T, Ndanguza D. Understanding correlates of child stunting in Ethiopia using generalised linear mixed models. BMC Public Health 2019;19(1):626. https://doi.org/10.1186%2Fs12889-019-6984-x [ Links ]
22. Fagbamigbe AF, Afolabi RF, Yussuf K, Adebowale AS, Yusuf BO. Unobserved heterogeneity in the determinants of under-five mortality in Nigeria : Frailty modeling in survival analysis. Afr J Appl Stat 2019;6(1):565-583. https://doi.org/10.16929/ajas/565.231 [ Links ]
23. Andersson N, Lamothe G. Clustering and meso-level variables in cross-sectional surveys: An example of food aid during the Bosnian crisis. BMC Health Serv Res 2011;11((S2):S15). https://doi.org/10.1186/1472-6963-11-s2-s15 [ Links ]
24. Chirande L, Charwe D, Mbwana H, et al. Determinants of stunting and severe stunting among under-fives in Tanzania: Evidence from the 2010 cross-sectional household survey. BMC Pediatr 2015;15(1):165. https://doi.org/10.1186/s12887-015-0482-9 [ Links ]
25. Masibo PK. Trends and determinants of malnutrition among children age 0-59 months in Kenya (KDHS 1993, 1998, 2003 and 2008-09). Calverton, MD, USA: ICF International; 2013. https://dhsprogram.com/pubs/pdf/WP89/WP89.pdf (accessed 13 December 2019). [ Links ]
26. Habyarimana F. Key determinants of malnutrition of children under five years of age in Rwanda: Simultaneous measurement of three anthropometric indices. Afr Popul Stud 2016;30(2):2328-2340. https://doi.org/10.11564/30-2-836 [ Links ]
27. Akombi B J, Agho KE, Hall JJ, Wali N, Renzaho AMN, Merom D. Stunting, wasting and underweight in sub-Saharan Africa: A systematic review. Int J Environ Res Public Health 2017;14(8):863. https://doi.org/10.3390/ijerphl4080863 [ Links ]
28. Malako GB, Asamoah BO, Tadesse M, Hussen R, Gebre MT. Stunting and anemia among children 6-23 months old in Damot Sore district, Southern Ethiopia. BMC Nutr 2019;5(1):3. https://doi.org/10.1186/s40795-018-0268-1 [ Links ]
29. Chirwa EW, Pe Ngalawa H. Determinants of child nutrition in Malawi. S Afr J Econ 2008;76(4):628-640. https://doi.Org/10.1111/j.1813-6982.2008.00212.x [ Links ]
30. Nshimyiryo A, Hedt-Gauthier B, Mutaganzwa C, et al. Risk factors for stunting among children under five years: A cross-sectional population-based study in Rwanda using the 2015 Demographic and Health Survey. BMC Public Health 2019;19(1):175. https://doi.org/10.1186/sl2889-019-6504-z [ Links ]
31. Danaei G, Andrews KG, Sudfeld CR, et al. Risk factors for childhood stunting in 137 developing countries: A comparative risk assessment analysis at global, regional, and country levels. PLoS Med 2016;13(11):e 1002164. https://doi.org/10.1371/journal.pmed.1002164 [ Links ]
32. Bain LE, Awah PK, Geraldine N, et al. Malnutrition in Sub-Saharan Africa: Burden, causes and prospects. Pan Afr Med J 2013;15(1):120. https://doi.org/10.11604%2Fpamj.2013.15.120.2535 [ Links ]
33. Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382(9890):427-451. https://doi.org/10.1016/S0140-6736(13)60937-X [ Links ]
34. Fenske N, Burns J, Hothorn T, Rehfuess EA. Understanding child stunting in India: A comprehensive analysis of socio-economic, nutritional and environmental determinants using additive quantile regression. PLoS ONE 2013;8(11):e78692. https://doi.org/10.1371/journal.pone.0078692 [ Links ]
35. Thorne CJ, Roberts LM, Edwards DR, Haque MS, Cumbassa A, Last AR. Anaemia and malnutrition in children aged 0-59 months on the Bijagós Archipelago, Guinea-Bissau, West Africa: A cross-sectional, population-based study. Paediatr Int Child Health 2013;33(3):151-160. https://doi.org/10.1179/2046905513Y.0000000060 [ Links ]
36. De Onis M, Branca F. Childhood stunting: A global perspective. Matern Child Nutr 2016;12(Suppl 1):12-26. https://doi.org/10.1111%2Fmcn.12231 [ Links ]
37. Le Cessie S, Verhoeff FH, Mengistie G, Kazembe P, Broadhead R, Brabin BJ. Changes in haemoglobin levels in infants in Malawi: Effect of low birth weight and fetal anaemia. Arch Dis Child Fetal Neonatal Ed 2002;86(3):F182-F187. https://doi.org/10.1136/fn.86.3.f182 [ Links ]
38. Smith LC, Haddad L. Reducing child undernutrition: Past drivers and priorities for the post-MDG era. World Dev 2015;68(1):180-204. https://doi.org/10.1016/j.worlddev.2014.11.014 [ Links ]
39. Haile D, Azage M, Mola T, Rainey R. Exploring spatial variations and factors associated with childhood stunting in Ethiopia: Spatial and multilevel analysis. BMC Pediatr 2016; 16(1):49. https://doi.org/10.1186/s12887-016-0587-9 [ Links ]
 Correspondence:
Correspondence:
R F Afolabi
rotimifelix@yahoo.com
Accepted 17 August 2020














