Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Child Health
versión On-line ISSN 1999-7671
versión impresa ISSN 1994-3032
S. Afr. j. child health vol.14 no.4 Pretoria dic. 2020
http://dx.doi.org/10.7196/SAJCH.2020.v14i4.1694
SHORT REPORT
HIV prevalence among HIV-exposed infants participating in a programme for the prevention of mother-to-child transmission at Albazine Health Centre in Maputo
A MoianeI; A E ChongoII; M N CucoIII; C FaielaIV
IBSc Hons; Department of Biological Sciences, Faculty of Science, Eduardo Mondlane University, Maputo, Mozambique
IIMSc; Department of Biological Sciences, Faculty of Science, Eduardo Mondlane University, Maputo, Mozambique
IIIBSc Hons; Department of Biological Sciences, Faculty of Science, Eduardo Mondlane University, Maputo, Mozambique
IVMPH; Department of Biological Sciences, Faculty of Science, Eduardo Mondlane University, Maputo, Mozambique
ABSTRACT
BACKGROUND. In 2002, Mozambique introduced an inclusive and complete programme for the prevention of mother-to-child transmission (PMTCT) of HIV, comprising several components of continuous healthcare.
OBJECTIVES. To describe HIV infection rates in HIV-exposed children participating in a PMTCT programme.
METHODS. The study comprised the retrospective collection of data of10 097 pregnant women and 2 269 HIV-exposed infants who attended the Albazine Health Centre in Maputo, Mozambique, between 2012 and 2015. Descriptive statistics were used to present HIV prevalence and compliance with antiretroviral therapy (ART) in pregnant women, as well as HIV prevalence in infants at the ages of 1 month and between 9 and 18 months.
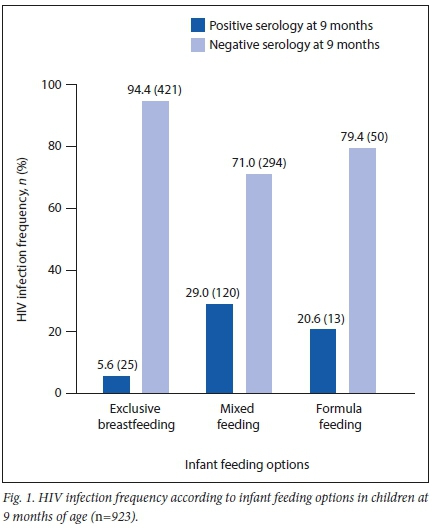
RESULTS. HIV testing coverage in pregnant women ranged from 84% in 2012 to 97% in 2015. HIV prevalence in pregnant women decreased from 16.7% in 2012 to 9.1% in 2015. Polymerase chain reaction (PCR) testing coverage in 1-month-old infants ranged from 62% in 2013 to 76% in 2015. Positive PCR results ranged from 4.4% in 2012 to 1.5% in 2015. The HIV prevalence in children between 9 and 18 months of age reached 6.8% in 2015. The HIV infection rate was lower (5.6%) among children who were exclusively breastfed than in those who received formula feeds (20.6%) or mixed feeding (29%).
CONCLUSION. Despite improvements in ART coverage, HIV prevalence in infants remains a cause of concern. Further studies should include following up mother-infant pairs to obtain a more accurate picture of ART adherence and infant feeding outcomes.
In Mozambique, the prevalence of HIV infection in children under 5 years of age was ~2.5% in 2016 and contributed 10% to infant mortality.Pl It is the third largest cause of morbidity after malaria and neonatal deaths.[1]
In children, mother-to-child transmission (MTCT) remains the most common form of HIV transmission, with 95% of infected children acquiring the virus through vertical transmission; the remaining 5% are infected through contaminated blood transfusions, rape and early sexual activity.[1]
Therefore, to control MTCT, it was important to introduce an inclusive and complete programme for the prevention of mother-to-child transmission (PMTCT), comprising several components of continuous healthcare, in Mozambique.[1] Initially (2002), only eight health centres offered antiretroviral therapy (ART) for pregnant women. However, the number of sites increased until the PMTCT programme acquired national status in 2004, and in 2006 a plan for accelerated expansion was introduced.[1] Given the economic situation, cultural context and other relevant factors, the health systems of countries implementing PMTCT programmes can select one of three options (A, B or B+) according to their respective advantages and disadvantages.[23] In 2013, Mozambique introduced Option B+, which simplified the provision of ART, standardised therapeutic regimes and ensured the availability of more effective prophylaxis for the PMTCT programme[1] With Option B+, pregnant women start ART as soon as they are diagnosed
as HIV-positive, regardless of gestational stage and CD4 count, and remain on lifelong ART.[23] With this option, infants receive nevirapine daily or azidothymidine twice a day from birth to age 4-6 weeks, regardless of feeding method.[2] Exclusive breastfeeding is recommended for the first 6 months of the infant's life irrespective of HIV status, after which complementary foods or drinks can be introduced. Breastfeeding should, however, continue till the age of 2 years or beyond.[4]
The B+ option offers several advantages, including greater efficiency and greater agreement with the Treatment 2.0 Framework for Action,[2] simplified and optimised use of ART and a standardised first-line treatment regimen. Moreover, it eliminates the necessity for multiple orientations and training.[2] However, disadvantages of the B+ option are that it needs to be evaluated in both programme and field scenarios, with an assessment of community acceptability and human rights protection.![2,3]
In 2011, Mozambique adopted the Global Plan for Elimination of MTCT of HIV and set its target to reduce MTCT to less than 5% by the year 2015;]1] the target date was subsequently extended to 2017.]1] The programme also envisaged 90% ART coverage to HIV-seropositive pregnant women.]1] Monitoring ART coverage and HIV infection rates in HIV-exposed children can identify trends and assist in providing valuable information that would enable programme managers to prioritise actions. The aim of the present study was to describe HIV infection rates in HIV-exposed infants participating in a PMTCT programme at the Albazine Health Centre (AHC) in Maputo, Mozambique.
Methods
Study setting
This descriptive study was conducted at the AHC in Maputo, Mozambique, which is a level 2 health unit located in the Albazine neighbourhood. It provides healthcare services to the neighbourhoods of Albazine, Magoanine C, Mutanhane and Guava.
Data collection
A retrospective analysis of health records was performed from a total of 10 097 pregnant women and 2 269 HIV-exposed infants for the period 1 January 2012 - 31 December 2015. The study was approved by the institutional ethics committee (Comité Institucional de Bioética em Saúde da Faculdade de Medicina & Hospital Central de Maputo), which waived the requirement for informed consent owing to the retrospective nature of the study. Only complete data sets of pregnant women were included in the study. The following indicators were collected: (i) HIV testing coverage in pregnant women; (ii) HIV prevalence in pregnant women; (iii) ART coverage in pregnant women; (iv) HIV testing coverage in children in the first month after birth and between 9 and 18 months of age; (v) HIV prevalence in children in the first month after birth and between 9 and 18 months of age; and (vi) infant feeding options and HIV prevalence.
On-site laboratory records, antenatal records and post-natal follow-up of HIV-exposed children were collected and evaluated. Secondary data used in this study were obtained from laboratory analysis of blood samples from pregnant women and infants born to HIV-infected women (HIV-exposed infants). In the case of 1-month-old children, blood was collected at the AHC and sent to the National Molecular Virology Laboratory of the National Institute of Health, as well as to the Molecular Virology Laboratory of the General Hospital of Mavalane for HIV diagnosis using polymerase chain reaction (PCR). For children aged 9 - 18 months and pregnant women, blood was collected and tested at the AHC using rapid tests (Alere Determine (Orgenics Ltd., Israel) and Uni-Gold Recombigen (Trinity Biotech PLC, Ireland)).
In relation to infant feeding options, an infant was classified as being exclusively breastfed, mixed-fed or formula-fed according to the mothers' responses as registered at the time of the PCR test. To describe the infant feeding option in relation to HIV infection, we included only serology data as obtained at 9 months of age. Pregnant women were tested for HIV infection on the day of opening their prenatal record; testing was not performed for those who claimed to be HIV-seropositive.
Descriptive statistics were used to explore HIV prevalence and ART compliance in pregnant women, as well as HIV prevalence in infants at the ages of 1 month and 9 - 18 months.
Results
HIV testing coverage in pregnant women ranged from 79.8% (2013) to 97% (2015) (Table 1). HIV prevalence decreased from 16.7% in 2012 to 9.1% in 2015. The ART coverage improved from 45.8% in 2012 to 100% in 2013 and the following years. PCR coverage in 1-month-old infants ranged from 62.4% to 76.7%, with the lowest coverage in 2013. Positive PCR ranged from 4.4% in 2012 to 1.5% in 2015 (Table 2).
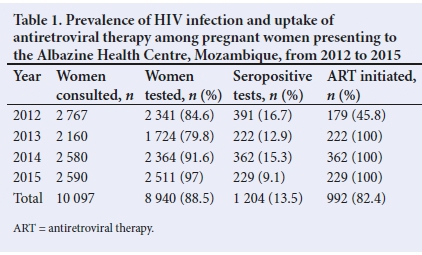
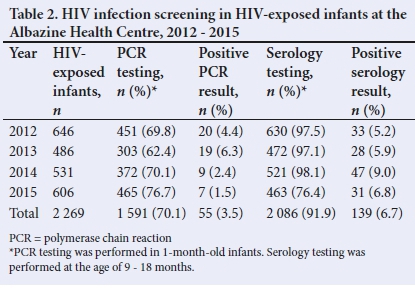
Despite good coverage and 90% serology in most years, HIV prevalence among children aged 9 - 18 months still ranged between 5% and 10%. The highest prevalence in this group (9%) was seen in 2014, followed by a decrease to 6.8% in 2015.
Infant feeding records (n=923) showed that the majority of mothers opted for exclusive breastfeeding rather than formula or mixed feeding (Fig. 1). The HIV infection rate was 5.6% for children who were exclusively breastfed, followed by a prevalence of 20.6% and 29% among formula-fed infants and those who received mixed feeding, respectively.
Discussion
Results showed that ART coverage improved from 45.8% in 2012 to 100% since 2013. This improvement may be linked to the B+ option of the PMTCT programme that was implemented in Mozambique in 2013. With this option, all HIV-positive pregnant women were eligible for ART once diagnosed. The reduction in viral load associated with ART consequently decreased the risk of vertical transmission.
HIV prevalence among infants of 1 month of age fell from 4.4% to 1.5% between 2012 and 2015. The B+ option and improved coverage may have contributed to the observed reduction in HIV infection rates post delivery. HIV prevalence among 9 -18-month-old children was 6.8% in 2015, which was still above the national target of 5%. To achieve the national and international goal for reducing MTCT, the AHC should explore how they can encourage women more effectively to adhere to the PMCT programme and consider performing PCR testing in infants in the first month of life, followed up with serology testing at 9 - 18 months. Monitoring ART adherence and the associated viral load during pregnancy and post partum may further improve the programme's effectiveness, especially to ensure suppression before delivery and during breastfeeding.
The HIV infection rate was lower among children who were exclusively breastfed, followed by those who received formula milk or mixed feeding. As we had no data concerning drug adherence in the context of 100% ART coverage nor on the number of women who achieved viral suppression, we were unable to test any possible association between infant feeding options and HIV infection rate. However, other studies[5,6] have shown that exclusive breastfeeding is associated with a lower frequency of HIV than in a mixed-feeding model.
As data from this study were not collected as mother-infant pairs, except for the infant feeding options, we were not able to correlate prevalence rates in infants with ART coverage.
Conclusion
Despite improvements in ART coverage, HIV prevalence in infants remains a cause of concern. Further studies should include following up infant-mother pairs for a more accurate understanding of ART adherence and infant feeding outcomes.
Declarations. This manuscript was submitted in partial fulfilment of the requirements for a Masters degree at the Eduardo Mondlane University. Acknowledgements. We would like to thank the staff of the Albazine Health Centre for providing the data for analysis.
Author contributions. All authors contributed equally to the research and manuscript development.
Funding. None.
Conflicts of interest. None.
References
1. Ministério da Saúde. Programma Nacional de Controlo de ITS, HIV/SAID. Relatório Anual das Actividades Relacionadas ao HIV/SIDA 2019. http://www.misau.gov.mz/index.php/relatorios-anuais (accessed 26 February 2020). [ Links ]
2. World Health Organization. The treatment 2.0 framework for action: Catalysing the next phase of treatment, care and support. Geneva: WHO, 2011. https://www.unaids.org/sites/default/files/media_asset/20110824_JC2208_outlook_treatment2.0_en_3.pdf [ Links ]
3. Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012:379(9832):2151-2161. https://doi.org/10.1016/s0140-6736(12)60560-1 [ Links ]
4. Decker S, Rempis E, Schnack A, et al. Prevention of mother-to-child transmission of HIV: Postpartum adherence to Option B+ until 18 months in Western Uganda. PLoS One 2017;12(6):e0179448. https://doi.org/10.1371/journal.pone.0179448 [ Links ]
5. Children & Aids. Mozambique Tratamento Antiretroviral. https://www.childrenandaids.org/Mozambique_Tratamento-Antiretroviral_2016 (accessed 25 November 2019). [ Links ]
6. Anoje C, Aiyenigba B, Suzuki C, et al. Reducing mother-to-child transmission of HIV: findings from an early infant diagnosis program in south-south region of Nigeria. BMC Public Health 2012:12:184. https://doi.org/10.1186/1471-2458-12-184 [ Links ]
 Correspondence:
Correspondence:
A E Chongo
alda.chongo@uem.ac.mz
Accepted 30 April 2020














