Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.13 n.4 Pretoria Dec. 2019
http://dx.doi.org/10.7196/sajch.2019.v13i4.1605
RESEARCH
Epidemiological trend of post-neonatal tetanus in a Nigerian teaching hospital
O B OgunfoworaI;T A OgunlesiII;O O Oba-dainiIII
IMBBS, FWACP; Consultant paediatrician, Department of Paediatrics, College of Health Sciences, Olabisi Onabanjo University, Sagamu, Nigeria
IIMB ChB, FWACP, FRCPCH; Consultant paediatrician, Department of Paediatrics, College of Health Sciences, Olabisi Onabanjo University, Sagamu, Nigeria
IIIMB ChB; Department of Paediatrics, Olabisi Onabanjo University Teaching Hospital, Sagamu, Nigeria
ABSTRACT
BACKGROUND. In sub-Saharan Africa, tetanus is one of the causes of childhood deaths with public health significance.
OBJECTIVE. To determine the current epidemiological trend of post-neonatal tetanus (PNT) in a Nigerian tertiary health facility.
METHODS. A retrospective study was done of the cases of tetanus (patients of age 28 days to 15 years) managed at the Olabisi Onabanjo University Teaching Hospital, Sagamu, south-west Nigeria, between January 2010 and December 2017.
RESULTS. There were 67 cases of PNT out of 3 171 admissions over the study period. The annual prevalence rates ranged from 3.9% in 2010 to 1.2% in 2017. The majority of patients were aged 6-12 years (55.2%) and 64.2% male and fell into the lower socioeconomic classes IV and V (98.4%). The mean (SD) duration of illness was 3.1 (2.2) days while the mean incubation period was 10.4 (5.4) days. The portal of entry was identifiable among 54 (88.5%) children. Most patients were not immunised against tetanus (45; 73.8%), had an incubation period >1 week (30; 49.2%), period of onset >24 hours (29; 47.5%) and severe and very severe disease (35; 57.4%).The case fatality rate was 35.8%, contributing 12.6% of total childhood deaths. Death was significantly associated with duration of illness less than 24 hours (p=0.032) and severe and very severe cases (p=0.005).
CONCLUSION. Although the prevalence rates of PNT declined over the 8-year study period, the disease still contributed major proportions of post-neonatal childhood deaths from unmet intensive care needs among severe cases.
Tetanus is a preventable, infectious, but non-contagious neurological disease associated with high mortality[1] It is characterised by uncontrolled muscular contractions as a result of the activity of a neurotoxin produced by the causative microbe, Clostridium tetania Although the pathogenesis and clinical symptomatology of tetanus are essentially the same irrespective of age, tetanus affecting newborn infants (neonatal tetanus (NNT)) is traditionally regarded as a clinical entity different from tetanus affecting older children (post-neonatal tetanus (PNT)). Available literature on paediatric tetanus appears to be highly skewed towards NNT, with few studies on PNT, presumably owing to the global health relevance of NNT as an index of the quality of prenatal and obstetric care available to pregnant women, especially in the developing world.[3]
Apart from measles, tetanus is reported to be the highest contributor to childhood mortality among the vaccine-preventable diseases.[1] While the USA reported a dramatic decline in the incidence of tetanus between 1900 and 2015, with only 29 cases reported in 2015,[4] such robust data are not available for the developing world. However, of the 36 086 deaths recorded from PNT globally in 2015, 47% occurred in South Asia, 36% in sub-Saharan Africa and 12% in south-east Asia.[5] Between 1990 and 2015, global mortality rates owing to PNT dropped by 81%. The 2015 PNT mortality rates were 0.75/100 000 for West Africa, 1.03/100 000 for South Asia and 0.10/100 000 for North Africa and the Middle East.[5] The PNT mortality rate in Nigeria dropped from 2.76/100 000 in 1990 to 0.70/100 000 in 2015.[5]
Over the preceding 20 years, few studies had been carried out on PNT in various parts of Nigeria[6-11] and other parts of the developing world.[12-14] Although NNT had been studied in Olabisi Onabanjo University Teaching Hospital (OOUTH), Sagamu - the premier state government-owned teaching hospital in Nigeria - with data collected over a 15-year period (1991 - 2005),[15] there has been no study of PNT in this centre.
There have been several globally co-ordinated efforts at eliminating maternal and neonatal tetanus in the few developing countries where tetanus is still a public health problem, such as Nigeria, India and Afghanistan.[16] These efforts include improved anti-tetanus vaccination and clean birth practices which have led to a progressive drop in the incidence of NNT in the developing world. The scenario appears different with PNT, which receives less global attention and seems neglected. Consequently, the present study was conducted with the aim of determining the current epidemiological trend (prevalence, clinical profile and predictors of poor outcome) of PNT at the OOUTH.
Methods
This was a retrospective study of the cases of PNT (patient ages 28 days to 15 years) managed at the children's ward of the OOUTH, Sagamu, south-west Nigeria, between January 2010 and December 2017. Official consent to use the data was obtained from the Health and Research Ethics Committee of the hospital.
Children with tetanus are admitted into the Children's Ward where infectious diseases are managed by at least two consultants, resident doctors, nurses and physiotherapists. The hospital does not have a paediatric intensive care unit, hence tetanus cases are nursed in dark and quiet isolation rooms with frequent oropharyngeal suction and other supportive care. Airway management is done by insertion of an oro-pharyngeal airway and frequent oro-pharyngeal toileting, while autonomic dysfunction is managed symptomatically. The medications used to control spasms include parenteral diazepam (5 -10 mg/kg/day), chlorpromazine (5 mg/kg/day) and phenobarbitone (5 mg/kg/day) administered every 6 hours, i.e. the medications are given in rotation in 2-hourly phases. Bovine anti-tetanus serum (5 000 IU) is administered intramuscularly and intravenously, while parenteral metronidazole is administered at a dose of 7 mg/kg every 8 hours. Surgical debridement was carried out by the surgical team if the portal of entry was a surface wound.
Study population
For postnatal-age children with a clinical diagnosis of tetanus, PNT was defined as acute illness characterised by muscle spasms and generalised hypertonia.[2] Each child with tetanus was classified as mild, moderate, severe and very severe, using the Ablett classification.[17]
Data collection
The hospital numbers, names and sex of children admitted with a diagnosis of PNT within the study period, and the dates of admission and outcome of hospitalisation, were obtained from the admissions and discharge register of the children's ward. The available hospital records (case files) of the children were retrieved from the Health Information Department of the hospital.
The data recorded for each child included place of residence of the family, highest education and present occupation of parents, and immunisation history. Socio-economic status of the family was determined by using the highest education and present occupation of the parents, as recommended.[18] Socio-economic classes were described as Classes I (the highest) to V (the lowest). Other data included details of clinical presentation such as duration of illness, portal of entry, incubation period, spectrum of presenting symptoms and signs and complications.
Data management
Data management was done with the Statistical Package for Social Sciences (SPSS) version 21.0 statistical software using simple descriptive statistics. The yearly prevalence of PNT was determined and groups of children were compared using the Pearson chi-square test with Yates' correction applied as necessary. Pearson correlation (r) analysis was performed to relate annual admissions to annual prevalence of PNT. The direct cost of care for each subject was converted from Naira (N) to US dollar ($) using the highest foreign exchange rate per year for the period of study. Statistical significance was determined by p-values <0.05.
Results
A total of 3 986 children were admitted into the children's wards (excluding the neonatal ward) over the study period. Sixty-seven cases of PNT were identified on the ward's admission register but the records of only 61 (91.0%) were available for retrieval and analysis.
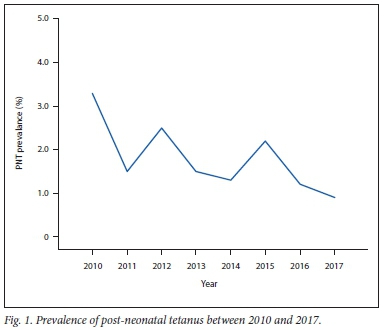
The overall prevalence rate of PNT among hospitalised children was 1.7% (67/3 986) but the annual prevalence rates ranged from 3.3% in 2010 to 0.9% in 2017, as shown in Fig. 1. The prevalence rates had a downward trend, although there were increases in 2012 and 2015 (r= -0.459, p = 0.253).
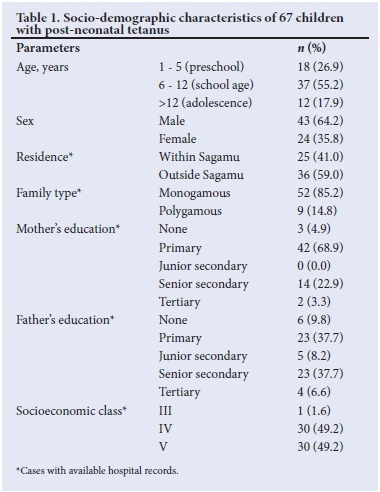
Ages ranged between 12 months and 16 years with a mean (SD) of 8.7 (4.2) years. There were 43 (64.2%) boys and 24 (35.8%) girls, giving a male:female ratio of 1.8:1. Table 1 shows the socio-demographic features of the initial 67 children; the majority were aged 6-12 years (37; 55.2%), resided outside Sagamu (36; 59%), belonged to a monogamous family setting (52; 85.2%), had poorly educated parents (mothers; 73.8% and fathers; 55.7%) and belonged to the lower socioeconomic classes IV and V (60; 98.4%).
The duration of illness before presentation at the hospital ranged between 1 day and 8 days with a mean of 3.1 (2.2) days, whilst the mean (SD) incubation period was 10.4 (5.4) days (range 3-21 days). Most of the 61 children with available records presented after 24 hours of illness (47; 77.0%), had an incubation period >1 week (30; 49.2%) and a period of onset >24 hours (29; 47.5%) (Table 2).
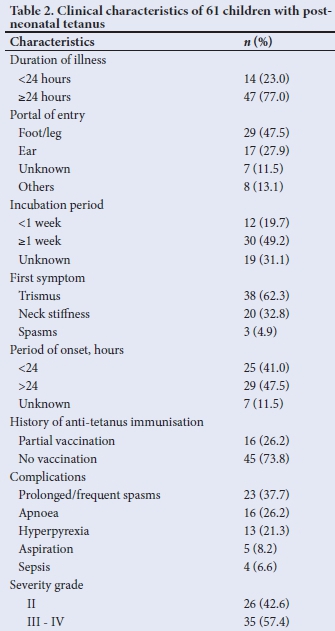
The portal of entry was identifiable among 54 (88.5%) children. The most common portal included wounds on the feet and legs (from puncture injuries, lacerations and fractures) (29; 47.5%); and ear discharges (17; 27.9%). The portal of entry was unidentified in 7 (11.5%) cases. Forty-five (73.8%) children were not immunised against tetanus, while 16 (26.2%) were partially immunised. None of them had booster doses of tetanus toxoid as scheduled. Trismus (38; 62.3%) was the most frequent presenting feature; prolonged or frequent spasm (23; 37.7%) was the leading complication; and 35 (57.4%) had severe and very severe disease.
Overall, the outcomes of hospitalisation among 67 children with PNT included discharge in good condition, death and discharge against medical advice (DAMA) among 37 (55.2%), 24 (35.8%) and 6 (9.0%) children, respectively. PNT deaths formed 12.6% of all childhood deaths in the children's ward (excluding the neonatal ward) and this ranged from 0.0% to 22.9% on an annual basis, as shown in Table 3. With the exclusion of the 6 children whose outcome was DAMA, the overall case fatality rate (CFR) was 39.3% (24/61) while the annual CFR (excluding the DAMA cases) ranged from 0.0% to 57.1% without a consistent yearly pattern of distribution (Table 3). The CFRs (with the exclusion of DAMA cases) were 35.7% (5/14), 41.9% (13/31) and 40.0% (4/10) among children aged <5 years, 6-12 years and >12 years, respectively The CFRs (with the exclusion of DAMA cases) among children with full records and with moderate and severe/very severe disease were 2/24 (8.3%) and 18/31 (58.0%) respectively, (χ2=12.388; p<0.001 with Yates' correction). The CFR was 37.0% among the children with identifiable portals of entry (20/54) and 28.6% (2/7) among those without identifiable portals of entry.
The overall duration of hospitalisation varied from 1 to 50 days with a mean (SD) of 14.7 (11.2) days. Forty-eight (71.6%) of the 61 children survived beyond the first week of hospitalisation. The mean (SD) cost of hospitalisation was USD109.4 (66.1) (range US$50.5 - 122.3) for the entire period of hospitalisation.
Table 4 depicts the relationship between outcome of hospitalisation and socio-clinical profile of children with PNT. The proportion of children who died was significantly higher compared with survivors in terms of residence outside Sagamu (p=0.013), with duration of illness <24 hours (p=0.032) and among those who had severe/ very severe disease (p=0.005). However, the two groups were comparable in terms of age distribution, sex, ear as portal of entry, foot or leg as portal of entry, period of onset and incubation period, and level of parental education.
Discussion
The prevalence rate of PNT in an institution without intensive care facilities over the 8-year period of study was 1.7%, which implied that about 1 out of every 40 hospitalised children had PNT. This prevalence rate was not remarkably different from the 1.1%[7] and 2.7%[9] prevalence rates previously reported from other southern Nigeria centres. It is difficult to estimate population-level prevalence of PNT for children aged < 15 years in the present study as the facility received children from diverse communities within and outside the state.
The prevalence rate observed in the present study may be low but it is still not acceptable, as tetanus is a vaccine-preventable disease with the possibility of being eliminated.[19] However, it is important to note that the prevalence rates recorded in the present study declined over time. Although this trend did not reach statistical significance, the annual prevalence of PNT was negatively correlated with the annual total admissions, suggesting a decline in the prevalence of PNT while the total admissions increased. This trend can only be explained in terms of improved preventive measures within communities rather than sequestration of cases in other health facilities, as no other centre in the catchment area served by OOUTH is equipped or staffed to manage severe childhood illnesses such as tetanus.
Children >5 years old constituted the bulk of the population studied, which is similar to previous Nigerian reports from Ibadan and Port Harcourt,[6,20] which may be attributed to the higher risk of injuries caused by unprotected environmental exposure during schoolgoing age. The preponderance of lower socio-economic status recorded in the present study affirms the known fact that tetanus tends to occur in the poorer strata of society, where risk of injury is high and access to quality healthcare is limited.[18] The preponderance of lower socioeconomic status in the present study was similar to previous reports from other parts of Nigeria.[5,6,8,9]
Although the portal of entry could not be identified among 11.5% of cases, the leading portals were wounds on the lower limbs and discharging ears in close to 75% of cases, which is similar to previous reports within[5,8,10,11] and outside[12,13] Nigeria. Interestingly, the present study revealed that pre-school-age children tended to have otogenic tetanus while lower-limb injuries were the predominant portals of entry among older children. It is important to stress that the inability to identify the portal of entry in some cases may pose challenges in the care of children with tetanus. This negates the usefulness of wound cleansing and debridement at the portal of entry as a way of reducing further toxin production by the causative organism.
Three-quarters of the children in this study were not immunised against tetanus, while the remaining quarter had partial immunisation. This observation was similar to previous reports of poor anti-tetanus vaccination status of children managed for childhood tetanus in parts of Nigeria.[6,9,11] These data stress the role of lack of, or poor, immunisation in the predisposition of children to tetanus. This situation is worsened by the fact that none of them received any booster dose of tetanus toxoid as scheduled in the routine immunisation programme.[19] In addition to making efforts to further improve coverage with the third dose of the diphtheria-pertussis-tetanus vaccine (DPT3) for Nigerian children from the reported 49% as at 2016,[1] it may also be important to advocate collaboration between the government agencies supervising health and education to ensure that booster doses of tetanus toxoid are routinely administered at primary school entry and exit.[6] While poor sero-conversion may explain the occurrence of tetanus in children with partial anti-tetanus immunisation, studies have also identified relatively high proportions of children who developed tetanus despite completed routine childhood immunisation.[10,14] While lack of booster doses of tetanus toxoid may explain this finding, further studies may be required to determine other immunological factors that may possibly prevent adequate sero-conversion following anti-tetanus immunisation.[19]
The overall CFR of 39.3% observed in the present study was similar to the 39.1% reported in eastern Nigeria,[11] but higher than the CFRs of 12%, 18%, 18%, 4.1% and 27.2% reported from Ibadan,[6] Calabar,[7] Maiduguri,[8] Lagos,[10] and Port Harcourt,[20] respectively which are all in Nigeria, compared with CFRs of 12.8% and 26.1% observed in India and Pakistan.[12,21] However, it is important to note the survival rate of 55.2% in our cohort in a setting which manages tetanus without intensive care facilities. Some other reports from paediatric intensive care units also reported CFRs ranging between 26.1% and 40% in India[13] and Pakistan,[21] respectively. Severe and very severe cases were significantly associated with mortality in the present study. This finding necessitates a call for the establishment of paediatric intensive care units in resource-poor parts of the developing world where childhood tetanus still occurs. Close to three-quarters of the children in this study survived beyond the first week of care; this raises the possibility that the quality of care in the first week of care may be critical to the survival of children with tetanus. Therefore, it is desirable to design adequately powered studies in the future to examine the factors which may influence outcome in childhood tetanus within the first week of hospitalisation.
Unfortunately, the average cost of care in the present study (US$109) for the entire period of hospitalisation was relatively high when compared with the funds available to an average family in the lower socio-economic class; this is a problem in a developing economy where the national monthly minimum wage is less than US$50. Most public sector-based healthcare services in Nigeria are subsidised as a matter of government policy and parents make out-of-pocket payment for these services. The mean cost of care of US$109 recorded in the present study was double the national minimum monthly wage of US$50 in the country. It is significant that the cost of care for a vaccine-preventable disease was double the national minimum wage when the vaccines are actually available free of charge to the population. This cost poses a great threat to the economic well-being of affected families. Therefore, efforts at improving DPT3 vaccine coverage, institution of routine administration of booster doses of tetanus toxoid and improved wound care should be intensified.
We acknowledge the retrospective design of the study as a limitation but the methodology can be applied in conducting a larger multi-centre study in the region.
Conclusion
Although the prevalence rates of PNT declined over the 8-year study period, the disease nevertheless contributed a major proportion of all childhood deaths in this resource-poor paediatric centre, especially from severe and very severe disease
Declaration. None.
Acknowledgments. The authors extend their appreciation to the members of staff of the Health Information Management Unit of the Olabisi Onabanjo University Teaching Hospital, Sagamu, Nigeria, for their assistance with data collection.
Authors' contributions. OBO and TAO conceived and designed the study. TAO and OOO collected and analysed the data while OBO and TAO interpreted the data. The manuscript was drafted by TAO while OBO did literature search and review. All the authors contributed to the intellectual content of the manuscript.
Funding. None.
Conflicts of interest. None.
References
1. World Health Organization. Immunization, Vaccines and Biologicals. Tetanus. www.who.int/immunization/monitoring_surveillance/burden/vpd/surveil-lance_type/passive/tetanus/en (accessed 25 February 2018). [ Links ]
2. Centers for Disease Control and Prevention. Tetanus (Clostridium tetani) 201C Case Definition, www.cdc.gov/nnds/conditions/tetanus/case-definition/2010/(accessed 25 February 2018). [ Links ]
3. Faulkner AE, Tiwari TS. Tetanus: Chapter 16.7. VPD Surveillance Manual. Centers for Diseases Control and Prevention. National Centre for Immunization and Respiratory Diseases. 2017. www.cdc.gov/vaccines/pubs/surv-manu-al/chptl6-tetanus.html (accessed 26 July 2018). [ Links ]
4. Kyu HH, Mumford JE, Stanaway JD, et al. Mortality from tetanus between 1990 and 2015; findings from the global burden of disease study. 2015. BMC Public Health 2017; 17( 1):719. https://doi.org/10.1186/sl2889-017-4111-4 [ Links ]
5. Ogunlesi TA. Vaccines for Women to prevent Neonatal Tetanus: RHL Commentary. Geneva: WHO Reproductive Health Library, 2011. [ Links ]
6. Fatunde JO, Familusi JB. Post-neonatal tetanus in Nigeria: The need for booster doses of tetanus toxoid. Niger J Paediatr 2001;28(2):35-38. [ Links ]
7. Anah MU, Etuk IS, Ikpeme OE, Ntia HU, Ineji EO, Archibong RB. Post-neonatal tetanus in Calabar, Nigeria: A 10-year review. Niger Med Pract 2008;54(2):45-47. [ Links ]
8. Alhaji MA, Akuhwa RT, Mustapha MG, et al. Post-neonatal tetanus in University of Maiduguri Teaching Hospital, North-Eastern Nigeria. Niger J Paediatr 2013;40(2):154-157. [ Links ]
9. Oyedeji OA, Fadero F, Joel-Medewase V Elemile P, Oyedeji GA. Trends in neonatal and post-neonatal tetanus admissions at a Nigerian teaching hospital. J Infect Dev Ctries 2012;6(12):847-853. https://doi.org/10.3855/jidc.2105 [ Links ]
10. Animashaun BA, Gbelee OH, Ogunlana AT, Njokanma OF, Odusanya O. Profile and outcome of patients with post-neonatal tetanus in a tertiary centre in South-West Nigeria; any remarkable reduction in the scourge? Pan Afr Med J 2015;21(1):254. https://doi.org/10.11604/pamj.2015.21.254.6488 [ Links ]
11. Chukwuka JO, Ezendu CE, Nnamani KO. Neonatal and post-neonatal tetanus in Nnamdi Azikiwe University Teaching Hospital, Nnewi, South-east Nigeria: A 10-year review. Trop J Med Res 2015;15( 1):30-33. https://doi.org/10.4103/1119-0388.152552 [ Links ]
12. Narang M, Choudhary N. Epidemiological trends of tetanus from East Delhi India: A hospital-based survey. J Infect Public Health 2014;7(2):121-124. https://doi.Org/10.1016/j.jiph.2013.07.006 [ Links ]
13. Angurana SK, Jayashree M, Bansal A, Singhi S, Nallasamy K. Post-neonatal tetanus in a PICU of a developing economy: Intensive care needs, outcome and predictors of mortality. J Trop Pediatr 2018;64(l):15-23. https://doi.org/10.1093/tropej/fmx020 [ Links ]
14. Tadele H. Clinical profile and outcome of pediatric tetanus: The experience of a tertiary hospital in Ethiopia. Ethiopian J Health Sei 2017;27(5):539-564. [ Links ]
15. Fetuga MB, Ogunlesi TA, Adekanmbi AF, Runsewe-Abiodun TI, Ogunfowora OB. Neonatal tetanus in Sagamu, Nigeria during the Expanded Programme on Immunization and National Programme on Immunization eras: A comparative analysis. Internet J Paediatr Neonatol 2010;12(1). [ Links ]
16. UNICEF. Elimination of Maternal and Neonatal Tetanus (Health). New York: UNICEF, 2013. www.unicef.org/health/index_43509.html (accessed 15 january 2015). [ Links ]
17. Cook TM, Protheroe RT, Handel JM. Tetanus: A review of the literature. Br ] Anaesth 2001;87(3):477-487. [ Links ]
18. Ogunlesi TA, Dedeke IOF, Kuponiyi OT. Socio-economic classification of children attending specialist health facilities in Ogun State. Niger Med Pract 2008;54:21-25. https://doi.org/10.4314/nmp.54il.28943 [ Links ]
19. Thwaites CL, Loan HT. Eradication of tetanus. Brit Med Bull 2015;116:69-77. https://doi.org/10.1093/bmb/ldv044 [ Links ]
20. Ide LEY, Uchenwa-Onyenegecha TA. Post-neonatal tetanus: 20 years experience as seen at the University of Port Harcourt Teaching Hospital. Brit J Med Med Res 2016; 12(2):1-5. https://doi.org/10.9734/BJMMR/2016/19047 [ Links ]
21. Naseem F, Mahar IA, Arif F. Two years study of tetanus cases in a paedi-atric intensive care unit. Pak J Med Sei 2016;32(3):641-645. https://doi.org/10.12669/pmjs.323.9165 [ Links ]
 Correspondence:
Correspondence:
T A Ogunlesi
tinuade_ogunlesi@yahoo.co.uk
Accepted 25 October 2018














