Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.13 n.4 Pretoria Dec. 2019
http://dx.doi.org/10.7196/sajch.2019.v13i4.1637
SHORT REPORT
Trends in diarrhoeal disease hospitalisation in a paediatric short - stay ward at a tertiary-level hospital in Soweto: 2002-2016
E MakgathoI; F PatelII; A IzuV, VI;M GroomeV, VI; S G LalaIV, VII;P VallabhIV;Z DangorIV, V, VI
IMMed; Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMSc, MMed Child Health, Neurodevelopment; Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIFCPaed; Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IVPhD; Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VPhD; Medical Research Council: Respiratory and Meningeal Pathogens Research Unit,Faculty of Health Sciences, University of the Witwatersrand: Johannesburg, South Africa
VIPhD; Department of Science and Technology/National Research Foundation: Vaccine Preventable Diseases, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VIIPhD; Perinatal HIV Research Unit, Faculty of Health Sciences, University of the Witwatersrand, JohannesburgSouth Africa
ABSTRACT
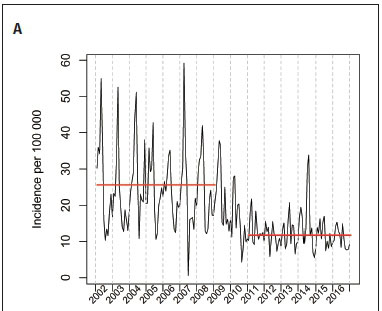
Globally, a decline in diarrhoeal disease has been observed over the last two decades. This may be attributable to several interventions, including rotavirus vaccination since 2009 in South Africa. From January 2002 to December 2016, we conducted a retrospective trend analysis of diarrhoeal hospitalisations with mild or moderate dehydration to a short-stay ward (SSW) in children <14 years of age. We found that diarrhoeal hospitalisation to the SSW accounted for 29.3% of the 53 717 children who presented with diarrhoea to the emergency department. A significant decline in disease incidence was noted after 2009/2010, coinciding with rotavirus vaccine implementation into the Expanded Programme on Immunisation. The yearly incidence (per 100 000) declined from 307 (95% confidence interval (CI) 277 - 337) over the 2002/2008 period to 141 (95% CI 120- 161) from 2011/2016; p<0.001. In conclusion, rotavirus vaccination was associated with a decline of diarrhoeal disease hospitalisations to the SSW at this hospital.
Diarrhoeal disease is a leading cause of disability-adjusted life-years (DALYs), morbidity and mortality worldwide, with an estimated 957 million cases reported in children <5 years of age in 2015.[1] Since the implementation of rotavirus vaccine, the global burden of rotavirus-associated and all-cause diarrhoeal disease has declined.[2] The effectiveness of rotavirus vaccination has been better for severe forms of rotavirus-specific diarrhoeal disease,[2] and marked reductions in diarrhoeal disease mortality rates (39.2%) and rotavirus-specific mortality rates (59.3%) have been observed worldwide.[13] In addition to introducing rotavirus vaccination into the Expanded Programme of Immunisation (EPI) to reduce the burden of diarrhoeal disease since 2009 in South Africa, there have been other interventions such as improved access to antiretroviral therapy (ART), the promotion of breastfeeding, safe Water Sanitation and Hygiene (WASH) initiatives, vitamin A supplementation and the Integrated Management of Childhood Illness (IMCI) programme (supplementary file available on request). The aim of the present study was to determine the trends and highlight time point changes to diarrhoeal disease hospitalisations, with mild and moderate dehydration, to a short-stay ward (SSW) at Chris Hani Baragwanath Academic Hospital (CHBAH) over a 15-year period (supplementary table available upin request).
Methods
A retrospective review of children of 6 weeks to <14 years of age with diarrhoea, hospitalised to a SSW at CHBAH from January 2002 to December 2016, was conducted. CHBAH is the third-largest hospital in the world, with 164 general paediatric beds and 40 short-stay paediatric beds. Until April 2014, when the primary-level Bheki Mlangeni District Hospital became operational, CHBAH was the only state-funded paediatric hospital in Soweto. The population of Soweto was estimated at 1.27 million in 2011, with approximately 314 000 children <14 years of age.[4] The population comprises mainly black people from low- to middle-income formal and informal households.
Children referred to CHBAH with a diarrhoeal disease are managed as per the IMCI protocol.[5] The standard of care for triage and management of children with a diarrhoeal disease at CHBAH has been mostly consistent over the study period. In addition, the senior Paediatric Medical Emergency and Ambulatory Department (PMEAD) medical staff have remained unchanged. Children >6 weeks of age and <14 years of age with mild or moderate dehydration are admitted to the SSW. Children <6 weeks of age are not routinely admitted to the SSW on the premise that they might have severe bacterial infection and require further investigation. Mild dehydration is defined as a child who is alert, drinks eagerly and has a normal urine volume. These children are hospitalised only if they do not tolerate oral fluids. Moderate dehydration is defined as a child with at least 2 of the following signs: sunken eyes, depressed anterior fontanelle, absent tears, thirst/drinks eagerly restless/irritable, a slow skin pinch but <2 seconds, and sticky mucosa. Excluded from admission to the SSW are children with: shock, suspected or proven electrolyte imbalances, decreased level of consciousness, a surgical cause of diarrhoea, chronic diarrhoea, the presence of other significant comorbidities (e.g. severe malnutrition, oncology patients, renal and cardiac disease) and those with no available caregiver, as the presence of a caregiver is mandatory in the SSW.
Monthly statistics using ward registries from the SSW and the PMEAD are routinely collected by senior medical staff. All children admitted to the SSW with a diagnosis of diarrhoea or having a discharge diagnosis of diarrhoea and/or dysentery were included in the study. Data were also available for the proportion of children requiring intravenous fluids for rehydration, and whether children resided in formal or informal housing. The incidence trends of diarrhoea with mild or moderate dehydration were estimated as the number of cases with diarrhoea admitted to the SSW over the median population estimates of children <14 years of age in Soweto and its surrounding areas as per Stats SA.[6] A non-parametric change point analysis (CPA) was undertaken to highlight changes to public health practices at various time points.[7] Unlike an intervention analysis, a CPA identifies points in time where a change in trend occurs. These points are referred to as change points. The change points are then associated with the public health intervention or strategies occurring around that change point. Average monthly minimum and maximum temperatures and rainfall patterns were obtained from Weather SA and seasonal patterns mapped out to determine associations between these parameters and diarrhoeal disease trends.[8] Permission to conduct the study was obtained from the University of the Witwatersrand Human Research Ethics Committee (M170501).
Results
Over half a million (N=503 512) children presented to the PMEAD over the 15-year period, and diarrhoeal disease occurred in 11.6% (n=53 717) (Table 1). The number of diarrhoeal cases seen in PMEAD for 2003 was missing and excluded from the calculations. We observed a 27.1% (12.5% in 2002 v. 9.1% in 2016) decline in the proportion of diarrhoeal disease consultations at the PMEAD. Although the number of children admitted with diarrhoeal illness to the SSW declined, the proportion of overall consultations remained unchanged (Table 1). Of the 17 057 children admitted to the SSW, 860 (5.0%) had dysentery. There was a 51.8% decline in the use of intravenous fluids for rehydration from 28.3% (n=418) in 2002 to 13.6% (n=92) in 2016. There was no difference in the formal or informal housing of patients over the 15years; 31.1% of parents reported living in informal settlements in 2002 compared with 28.2% in 2016 (p=0.447) (data not shown).
The CPA identified change points in monthly diarrhoeal incidence in May 2009 and July 2010. We then sought to determine the most likely intervention/s that may have been associated with that change point - rotavirus vaccine introduction into the EPI and changes to the prevention of the mother-to-child-transmission (PMTCT) programme were the interventions that began just prior to the change points identified (Fig 1A). Based on these results, we then compared the 2002 - 2008 (pre-rotavirus) with the 2011 - 2016 (post-rotavirus) vaccine period, and considered 2009 and 2010 as a transition period. Rotavirus vaccine coverage was estimated as 85% in 2011 and 91% in 2016.[9] During the 2002 - 2008 period, the average yearly incidence (per 100 000) was estimated at 307 (95% confidence interval (CI) 227 - 337), and declined to 141 (95% CI 120 - 161) over the 2011 - 2016 period (p<0.001) (Fig 1A). No other significant declines in relation to public health interventions (Table 1) were observed at other time points.
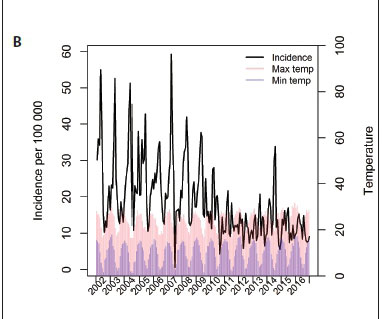
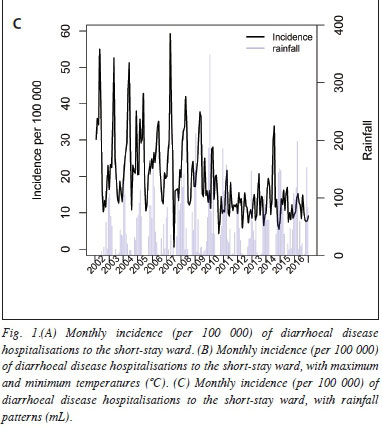
Average temperatures and rainfall patterns were plotted against the monthly diarrhoeal incidence rates (Figs 1B and 1C). Higher maximum and minimum temperatures were closely followed by peaks in diarrhoeal hospitalisations. Similarly, increased rainfall was associated with peaks in hospital admissions. These patterns were less distinct in the post-rotavirus vaccine era.
Discussion
To our knowledge, this was the first study to report long-term trends of hospitalisation for diarrhoeal disease with mild and moderate dehydration in South Africa (SA). Previously, a study conducted at CHBAH looked at trends in hospitalisations for more severe diarrhoeal disease.[10] We report a significant temporal decline in incidence of 'milder diarrhoeal disease that followed introduction of the rotavirus vaccine into the EPI in 2009 and persisted with vaccine coverage rates above 85%. Although other interventions, such as improved access to ART, PMTCT, WASH, IMCI, breastfeeding practices and vitamin A supplementation have probably contributed to this decline, the most marked change was observed after rotavirus vaccine implementation.
Numerous studies have reported the effectiveness of rotavirus vaccination on rotavirus-associated and all-cause diarrhoea in both high- and low-income settings.[2,10] The effectiveness has reportedly been less for milder forms of diarrhoeal disease.[2] Despite the 27% decline in the number of diarrhoeal patients seeking medical care at this PMEAD, our study did not include community-managed diarrhoeal disease. We were, however, able to show an effect on a specific subgroup of children with diarrhoeal disease who required primary- or secondary-level care for mild or moderate dehydration and, importantly, this once-busy gastroenteritis' unit in a high-burden setting may soon be redundant. Nonetheless, diarrhoeal disease remains a high disease burden in Africa, with marked variations in incidence and mortality,[11] and interventions to further reduce the burden of disease should continue.
Seasonal variations in diarrhoeal disease incidence are well described in the literature.[12] In SA, peaks occur in summer for bacterial pathogens and in winter for rotavirus infection.[13,14] A study conducted in Cape Town showed an increase in diarrhoeal disease incidence with an increase in ambient temperature.[15] In our study diarrhoeal hospitalisations to the SSW occurred mainly in the late summer months following peaks of temperature increases and rainfall patterns. However, we observed less effect of the above weather patterns after the implementation of the rotavirus vaccine.Similar findings have been reported elsewhere.[16]
The aforementioned public health initiatives may have had minor effects that occurred at other time points or were masked by the large impact of rotavirus vaccination, and were therefore not identified in the CPA. Additionally, improvements to the PMTCT and ART programmes may have influenced diarrhoeal disease hospitalisation in HIV-infected and HIV-exposed children; however, we did not have data on HIV status to determine the impact of these changes. Further limitations of this retrospective review were that we could not obtain all registries to verify the captured statistics because registration books were destroyed or misplaced over the years; however, statistics were consistently captured by the same senior medical staff over the entire study period. The ward statistics for the year 2003 were missing and therefore were excluded from any calculations. Furthermore, the incidence rates for diarrhoeal disease hospitalisations may have been underestimated, as children <6 weeks of age are excluded from admission to the SSW. Also, this study was conducted at only one hospital and therefore may not be extrapolated to other areas in SA.
Conclusion
Rotavirus vaccination was associated with a significant decline in the burden of diarrhoeal disease hospitalisations to a SSW in this hospital setting, and efforts to ensure adequate and continued supply of vaccines should continue. Further studies are needed in SA to further assess the relevance of dedicated diarrhoeal units in an era of widespread rotavirus vaccination.
Declaration. None.
Author contributions. Conception or design of the work: EM, FP PV and ZD; acquisition, analysis or interpretation of data: EM, AL ZD; drafting the manuscript: EM and ZD; revising the manuscript critically: FP, AI, MG, PV, SGL; final approval of the version to be published: EM, FP, AI, MG, SGL, PV, ZD.
Acknowledgements. We would like to acknowledge the ward 31 (PMEAD) and ward 39 (SSW) staff at the Chris Hani Baragwanath Academic Hospital.
Funding. None.
Conflicts of interest. None.
References
1. GBD Diarrhoeal Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis 2017;17(9):909-948. https://doi.org/10.1016/1473-3099(17)30276-1 [ Links ]
2. Yen C, Tate JE, Patel MM, et al. Rotavirus vaccines, update on global impact and future priorities. Hum Vaccin 2011;7( 12):1282-1290. https://doi.org/10.4161/hv.7.12.18321 [ Links ]
3. Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. Global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000 - 2013. Clin Infect Dis 2016;62(S2):S96-S105. https://doi.org/10.1093/cid/civl013 [ Links ]
4. Statistics South Africa. Census 2011. 2012. http://www.statssa.gov.za/ipage-id=4286&id=l1317 (accessed 14 March 2019). [ Links ]
5. World Health Organization. Integrated Management of Childhood Illness, Chart Booklet. Geneva: WHO, 2014; p. 1-80. https://www.who.int/maternal_child_adolescent/documents/IMCI_chartbooklet/en/ [ Links ]
6. Statistics South Africa. Gauteng-Sub-district Population Mid-year Estimates 2002-2016. Pretoria: Stats SA. [ Links ]
7. Ross GJ, Adams NM. Two nonparametric control charts for detecting arbitary distribution changes. J Qual Technol 2012;44(2):102-116. https://doi.org/10.1080/00224065.2012.11917887 [ Links ]
8. South African Weather Service. Statistics South Africa (STATS-SA). Monthly average minimum and maximum temperature and rainfall, 2002-2016. Pretoria; Stats SA, 2017. [ Links ]
9. WHO, UNICEE South Africa: WHO and UNICEF estimates of national immunization coverage: 2016 revision. 2017. Geneva: WHO, 2017. [ Links ]
10. Groome MJ,Zell ER, Solomon F, et al. Temporal association of rotavirus vaccine introduction and reduction in all-cause childhood diarrheal hospitalizations in South Africa. Clin Infect Dis 2016;62(S2):S188-S195. https://doi.org/10.1093/cid/civl204 [ Links ]
11. Reiner RC, Graetz N, Casey DC, et al. Variation in childhood diarrheal morbidity and mortality in Africa, 2000-2015. Ν Engl J Med 2018;379:1128-1138. https://doi.org/10.1056/NEJMoal716766 [ Links ]
12. Xu Z, Hu W, Zhang Y, et al. Exploration of diarrhoea seasonality and its drivers in China. Sei Rep 2015;5:824. [ Links ]
13. Robins-Browne RM. Seasonal and racial incidence of infantile gastroenteritis in South Africa. Am J Epidemiol 1984;119(3):350-355. [ Links ]
14. Mapaseka SL, Dewar JB, van der Merwe L, et al. Prospective hospital-based surveillance to estimate rotavirus disease burden in the Gauteng and North West Province of South Africa during 2003-2005. J Infect Dis 2010;202 Suppl:S131-S138. [ Links ]
15. Musengimana G, Mukinda FK, Machekano R, Mahomed H. Temperature variability and occurrence of diarrhoea in children under five years of age in Cape Town Metropolitan sub-districts. Int J Environ Res Public Health 2016;13(9):859:1-12. [ Links ]
16. Sanchez-Uribe E, Esparza-Aguilar M, Parashar UD, Richardson V. Sustained reduction of childhood diarrhea-related mortality and hospitalizations in Mexico after rotavirus vaccine universalization. Clin Infect Dis 2016;62(S2):S133-S139. https://doi.org/10.1093/cid/civl205 [ Links ]
 Correspondence:
Correspondence:
Z Dangor
ziyaad.dangor@wits.ac.za
Accepted 15 May 2019