Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.13 no.2 Pretoria Jun. 2019
http://dx.doi.org/10.7196/sajch.2019.v13i2.1574
CASE REPORT
Segmental atrophy of the liver in a child: Case report and review of the literature
A G BedadaI; M I SreekumaranII; G AzzieIII
IMD; Department of Surgery, Faculty of Medicine, University of Botswana, Princess Marina Hospital, Gaborone, Botswana
IIMD; Department of Surgery, Bokamoso Private Hospital, Gaborone, Botswana
IIIMD; Division of General and Thoracic Surgery, Hospital for Sick Children, Toronto, Canada
ABSTRACT
Segmental atrophy of the liver (SAL) is a pseudotumour that is extremely rare in children. We report the case of a 4-year-old child who presented with abdominal pain and a non-tender mass at the epigastrium. A computed tomography (CT) scan revealed a complex lesion of the left lobe of the liver. At laparotomy, a mass arising from the caudate lobe was completely excised and the pathology report confirmed SAL. Although rare in children, paediatricians, surgeons and radiologists should consider the possibility of SAL in the differential diagnosis of hepatic masses in this group of patients.
Segmental atrophy of the liver (SAL) is a rare and under-recognised hepatic pseudotumour.[4] It is usually single and may affect any hepatic lobe/segment.[1] The pathogenesis of SAL is not well understood but it is postulated to be related to a vascular injury.[1-3,5]
SAL is generally asymptomatic, but may present with upper-abdominal pain.[1] SAL may be found incidentally on ultrasound (US), computed tomography scan, magnetic resonance and even intraoperatively.[1-31 On US, the lesion can be iso-, orhypoechoic,[1, 4] with ill-defined margins and no detectable colour Doppler flow.[1] On contrast-enhanced CT, it appears as a well-circumscribed, hypodense, non-enhancing liver mass.[1, 4,-6] It may be partially calcified.[1] SAL may also mimic metastases on diagnostic imaging.[1,4] Its lack of fludeoxyglucose (FDG) uptake on positron emission tomography/computed tomography (PET/CT) may be due to the benign nature of the lesion.[1]
Histology alone can confirm the diagnosis of SAL.[3] In its early phases, SAL shows collapsed parenchyma, focal elastotic changes, and brisk bile ductular proliferation. Later in its course, elastotic change is seen. In its final stages, SAL demonstrates formation of elastotic nodules and dense fibrosis.[1-4] Because of its combined clinical and pathological peculiarities, SAL is the most under-recognised pseudotumour[13]
Case
A 4-year-old girl, weighing 16.7 kg, presented with a 2-year history of abdominal pain and a mass. The patient had no history of trauma. She had no significant past medical history related to the liver or biliary tree. There was no history suggestive of thrombotic disorders in the family.
On examination, she was thin, but not emaciated. She had no jaundice. Her vital signs were within normal limits. Abdominal examination revealed a -10 cm x 10 cm, non-tender, epigastric mass which moved with respiration. The origin of the mass was difficult to discern, but the sense was that it was related to a normal-sized liver. She had no splenomegaly.
Laboratory results revealed the following: haemoglobin level, 11.6 g/dL; white blood cell count, 7.16 x 103/μί; platelet count, 436 x 103/μί. Her Na+, K+, and CL were within normal limits. Her international normalised ratio (INR) was 0.99, aspartate aminotransferase (AST) 20.1 IU/L, alanine transaminase (ALT) 8.6 IU/L, GGT 37.6 IU/L, albumin 46.56 g/L, total bilirubin 1.4 U/L and direct bilirubin <1.2μmol/ί. Her creatinine was 35 μmol/L, and her urea was 4.6 mmol/L. Her serum alpha-fetoprotein was within normal limits, and her indirect haemagglutination test for hydatid disease was negative. The ultrasound revealed a complex mass in the left lobe of the liver. The CT scan demonstrated a 9 cm x 10 cm liver lesion with central hypo- and peripheral iso-dense areas. The radiologists listed differential diagnoses that included benign and malignant liver lesions, and even included hydatid disease of the liver (Fig. 1A).
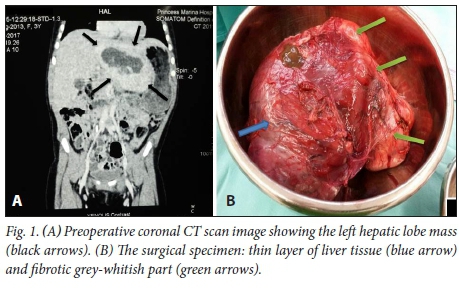
Given the symptomatology and findings, the patient was prepared for elective operation. The sense was that we were dealing with either a benign or a malignant primary liver tumour. The uncharacteristic imaging as well as the negative serology for hydatid disease, while not absolutely ruling out the diagnosis, made this entity much less likely. At laparotomy, a solid 10 cm x 10 cm mass involving most of the caudate lobe of the liver was found. The mass appeared well vascularised, and alternated in colour from brown to whitish-grey. The tumour was completely excised and sent for pathological analysis (Fig. 1B). One big vein arising from the mass and draining directly to the inferior vena cava was ligated. The remainder of the liver and all other intra-abdominal organs were normal. As a precaution, the patient was kept in the intensive care unit for 24 hours postoperatively. Her course was unremarkable, and she was discharged home on the 12th postoperative day.
The pathology result described an encapsulated 9.5 cm x 7 cm x 2.5 cm segment of liver, the cut section of which revealed a poorly circumscribed area of whitish fibrous tissue intermixed with semisolid gelatinous tissue. The surrounding liver parenchyma had areas of dark yellow tissue intermixed with similar white fibrous tissue. Microscopic examination revealed areas of elastosis to fibrosis and associated small islets of hepatocytes. At the periphery, there was liver parenchyma with fibrous tracts showing mild bile duct proliferation. Thin-walled veins were present in the fibrous septa. In the areas of fibrosis, there were a few vessels with prominent walls. The features were compatible with segmental atrophy of the liver (SAL), with no evidence of other pathological entities.
The patient remains well 6 months after the operation. A follow-up ultrasound done three months after the surgery did not show any evidence of residual/recurrent disease.
Discussion
SAL is an under-recognised benign lesion.[1-4] Though it is widely reported in adults[1,2,4,5,7] our review of the literature found only one paediatric patient: a 14-year-old.[5] The lesions are usually much smaller than anatomical segments and often involve only a few hepatic lobules.[1] Bile leakage from transected ducts resulting in inflammation and fibrosis and damage to the small peribiliary capillaries may cause SAL.[7] Larger SAL has been considered a complication of different benign and malignant diseases of the liver and of the bile ducts,[7] including hydatid disease, cholangiocarcinoma, alcoholic cirrhosis, chronic active hepatitis with cirrhosis, hepatocellular carcinoma, cryptogenic cirrhosis, pyogenic cholangitis, sclerosing cholangitis, and acute hepatic failureJ1,2] Benign stricture causes atrophy by biliary obstruction, while cholangiocarcinoma causes atrophy by biliary obstruction and/or portal vein branch compromised Histology remains the gold standard of diagnosis.
Garg et in their 6-case series reported that all 6 patients were female with a mean age of 58.3 years (range 37 - 80) and the mean size of the lesion 1.8 cm (range 3 - 3.6 cm). Singhi et al.[5] in their 18-case series found that the median age of presentation was 63 years (range 14 - 91), female patients constituting 72% of the cases; 78% of the cases presented with right upper quadrant abdominal pain, the size of the tumour ranged from 1.8 to 10 cm and 83% of the lesions were subcapsular.[1]
Our case of a 4-year-old girl is the youngest patient described in the literature. She presented with abdominal pain and a palpable mass. The lesion was located in the caudate lobe and it measured 9.5 cm x 7 cm x 2.5 cm.
There is no specific laboratory test or biomarker for SAL. The histological features of SAL seem to vary based on when the biopsy is taken: early in the course (first stage), the lesion is characterised by collapsed hepatic parenchyma with associated islands of residual hepatocytes, chronic inflammation and marked bile duct proliferation. Later in the course (second stage), histological findings are characterised by little to no ductal proliferation, a decrease of chronic inflammation and increased amount of elastosis. As time passes and later in the course (third stage), histological findings show that the lesion is almost entirely composed of elastotic fibres with scattered small islands of residual hepatocytes and portal tracts. In its latest stages (fourth stage), the lesion forms nodules with dense fibrosis.[1-5] Based on the histology in our patient, she seems to have presented in the second stage. As demonstrated in our case, complete excision remains the treatment of choice.[1,3]
Conclusion
SAL is a focal hepatic lesion that can mimic primary hepatic or metastatic lesions. It may present with abdominal pain and a mass. As demonstrated in our patient, paediatricians, surgeons and radiologists should be aware of this very rare condition, even in a child as young as 4 years of age. Histology remains the gold standard of diagnosis.
Acknowledgements. None.
Author contributions. AGB reviewed the literature, followed the patient postoperatively and prepared the manuscript. MIS performed the operation, followed the patient until discharge, secured consent from the parents and reviewed the manuscript. GA reviewed the manuscript. All authors approved the manuscript for publication.
Funding. None.
Conflicts of interest. None.
References
1. Garg I, Graham RP, van Buren WM, Goenka AH, Torbenson MS, Venkatesh SK. Hepatic segmental atrophy and nodular elastosis: Imaging features. Abdom Radiol 2017;42(10):2447-2453. https://doi.org/10.1007/s00261-017-1164-x [ Links ]
2. Spolverato G, Anders R, Kamel I, Pawlik TM. Segmental atrophy of the liver: An uncommon and often unrecognized pseudotumor. Dig Dis Sci 2014;59(12):3122-3125. https://doi.org/10.1007/s10620-014-3231-2 [ Links ]
3. Bhaijee F, Oshima K, Anders RA. Hepatic mass lesions: Challenges and pitfalls. Diagnostic Histopathol 2017;23(12):544-552. https://doi.org/10.1016/j.mpdhp.2017.11.002 [ Links ]
4. Ishizaki Y, Mizuno T, Hara K, Kawasaki S. Advanced segmental atrophy of the liver with marked elastosis. Surgery 2015;157(4):826-827. https://doi.org/10.1016/j.surg.2013.10.021 [ Links ]
5. Singhi AD, Maklouf HR, Mehrotra AK. Segmental atrophy of the liver: A distinctive pseudotumor of the liver with variable histologic appearances. Am J Surg Pathol Mar 2011;35(3):364.371. https://doi.org/10.1097/PAS.0b013e31820b0603. [ Links ]
6. Friesen BR, Gibson RN, Speer T, Vincent JM, Stella D, Collier NA. Lobar and segmental liver atrophy associated with hilar cholangiocarcinoma and the impact of hilar biliary anatomical variants: A pictorial essay. Insights Imaging 2011;2(5):525-531. https://doi.org/10.1007/s13244.011-0100-9 [ Links ]
7. Kaushik S, Fulcher AS, Turner MA. Segmental hepatic atrophy: A sequela of blunt intrahepatic bile duct injury. J Trauma 2003;54(6):1225-1127. [ Links ]
 Correspondence:
Correspondence:
A G Bedada
bedaleOO@yahoo.co.uk
Accepted 17 September 2018














