Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.13 no.2 Pretoria Jun. 2019
http://dx.doi.org/10.7196/sajch.2019.v13i2.1544
RESEARCH
The quality of life of caregivers of children with atopic dermatitis in a South African setting
B SinghI; Y ThandarII; Y BalakrishnaIII; A MosamIV
IMB ChB; Department of Dermatology, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIBPharm, MMedSc, PhD; Department of Basic Medical Sciences, Faculty of Heath Sciences, Durban University of Technology, Durban, South Africa
IIIMSc (Statistics); Biostatistics Unit, South African Medical Research Council, Durban, South Africa
IVMB ChB, FCDerm, MMed, PhD; Department of Dermatology, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND. Atopic dermatitis (AD) is known to adversely affect patients' quality of life (QOL). However, less is known about the extent to which caregivers are affected, particularly in developing countries.
OBJECTIVES. To investigate factors affecting QOL in caregivers of children with AD in the South African (SA) setting and to document the associated effect of disease severity.
METHODS. This was a prospective study of 142 AD patients and their caregivers attending Grey's Hospital in KwaZulu-Natal, SA, between May and September 2016. Disease severity was assessed according to the Objective Scoring of Atopic Dermatitis (Objective SCORAD) index. The Dermatitis Family Impact (DFI) questionnaire was used to assess QOL.
RESULTS. The study population included 119 (84%) black, 20 (14%) Indian and 3 (2%) coloured patients. Among the group, 44% of cases (n=62) were classified as mild, 53% (n=76) as moderate and 3% (n=4) as severe. The DFI score was significantly associated with the Objective SCORAD index (p<0.0001). QOL factors significantly affected were emotional distress of the caregiver (p<0.0001), tiredness of the caregiver (p<0.0001) and family leisure activities (p<0.0001). Involvement in treatment (p=0.016), food preparation and feeding (p=0.003), the family's sleep (p=0.001) and the caregiver's relationships (p=0.025) were moderately affected.
CONCLUSION. The QOL of caregivers of children with AD in this setting was adversely affected and declined with increasing disease severity. An evaluation of the psychosocial health of caregivers and appropriate referral where necessary are important for holistic management of both the patient and the caregiver and to improve disease outcome.
Atopic dermatitis (AD) is a chronic, relapsing inflammatory skin disease. It is strongly associated with a family and personal history of other atopic conditions, such as asthma and allergic rhinitis. Besides genetic susceptibility, external and lifestyle factors are critical to the development of atopic diseases.[1]
Most AD studies have focused on children. There remains a paucity of data on the prevalence of AD in South Africa (SA).P[3] Global data from the International Study of Asthma and Allergies in Childhood (ISAAC)[4] show that eczema is a major problem in developing countries. ISAAC Phase Three showed a 13.3% prevalence of AD symptoms in children aged 13 - 14 years in Cape Town, up from 8.3% in Phase One.[4] Centres of high prevalence for AD symptoms appear common in Africa, with a total prevalence of 12.3% in SA.P[5] A study of Xhosa children[6] further demonstrated a clear urban-rural gradient for the occurrence of AD in black children. Much of the current information on quality of life (QOL) in AD has come from scales developed in Western cultures, with very few documented studies from other ethnic groups.[7]
AD undoubtedly has a profound effect on the child. However, little attention is given to the emotional wellbeing of the caregiver and the possible burden of caring for a child with AD. Acknowledging this is essential in offering appropriate care. Interventions merely to reduce AD symptoms are insufficient to improve disease management by caregivers and the psychological and familial situation must be considered.[8] Studies in the UK, Europe, USA, Australia and Saudi Arabia[7-11] confirm that an increase in AD severity negatively affects the QOL of parents as primary caregivers, which, in turn, negatively affects the child's health. In SA, the parenting role is often the responsibility of grandparents, aunts, uncles and, occasionally, siblings. To our knowledge, there are currently no published data on QOL of either AD patients or their caregivers in SA. The present study investigates the effect of childhood AD on caregivers in an SA population.
Methods
Caregivers of children with AD aged 16 years or younger were invited to participate. The study was conducted at the outpatient dermatology clinic at Grey's Hospital, a tertiary-level hospital in Pietermaritzburg, SA. Consecutive cases that met the specified criteria were collected between May and September 2016. The participant had to be the person primarily responsible for caring for the patient, allowed to legally consent for the patient and be literate in English or isiZulu. Informed consent was required for participation.
Patients were diagnosed with AD based on the UK (Atopic Dermatitis Diagnostic Criteria) Working Party's criteria for eczema[12] or clinical assessment by the attending dermatologist.
Caregivers who declined participation or who did not meet the inclusion criteria were excluded. Confidentiality was assured. Participants could decline participation or withdraw at any point without reasons or repercussions. No attempts were made to influence the study in any way by alterations to or discontinuation of treatment.
An interview guide to record the patient's and caregiver's sociodemographic details was used by all participating dermatologists. This included information regarding: the patient's age, gender, race, treatment duration, and other medical conditions. Caregivers' information included: relationship to the patient (e.g. mother), number of other dependants younger than 16, marital status, occupation, income, and medical history.
An examination to evaluate disease severity was performed using the Objective Scoring of Atopic Dermatitis (SCORAD) index, a validated clinical assessment tool grading AD severity based on extent and intensity of lesions. Severity scores were described as mild (<15), moderate (15 - 40) or severe (>40). The maximum possible score was 83, with 10 additional points allowed in the case of disfiguring eczema of the face or hands.[13]
Caregivers completed the Dermatitis Family Impact (DFI) questionnaire, a validated 10-item, disease-specific scale that assesses areas of daily life and family function and is completed specifically by the caregiver of a child with AD. [14] The response to questions was scored as follows: 'very much' (3 points), 'a lot' (2 points), 'a little' (1 point), 'not at all' (0 points). The final score ranged from 0 to 30.
The calculated disease severity was compared with DFI scores. Descriptive statistics such as means, frequencies and percentages were used to analyse data on socioeconomic circumstances.
All questionnaires and consent forms were translated to isiZulu by a translator accredited with the South African Translators' Institute. The material was back-translated by another accredited translator to verify its accuracy and cultural appropriateness for use in isiZulu.
Statistical analysis
Data were analysed using Stata 14 (StataCorp, USA). The association between the DFI score and AD severity was described according to the Pearson correlation coefficient. Spearman rank correlation was used to describe the association between the DFI score and number of dependants. The difference in DFI scores between AD severity categories was assessed using a one-way analysis of variance. Associations between DFI scores and categorical variables were investigated using the Kruskal-Wallis test. Results with a p-value <0.05 were considered statistically significant. The Cronbach's alpha statistic was used to measure internal consistency of the DFI questionnaire.
Ethical considerations
Ethical approval was attained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (ref. no. BE097/16).
Results
Characteristics of study population
A total of 142 caregivers of AD patients and who met the inclusion criteria were approached and agreed to participate. Table 1 describes the sociodemographic characteristics of caregivers and patients. The mean age of patients was 5 (4) years, with 79 (56%) being male and 63 (44%) being female. Of the total study population, 119 patients (84%) were black, 20 (14%) were Indian and 3 (2%) were coloured. AD was diagnosed as moderate in 53% (n=76) of cases, mild in 44% (n=62) and severe in 3% (n=4). The mother was the primary caregiver in 73% (n=103) of cases and 98 caregivers (69%) had at least one other dependant younger than 16 years living with them.
Dermatitis Family Impact questionnaire
A high Cronbach's alpha (0.92) suggested good internal consistency of the DFI questionnaire and indicated a reliable scale. The DFI mean (standard deviation (SD)) was 8.9 (7.2) for mild AD, 14.7 (7.6) for moderate AD and 22.3 (6.5) for severe AD. The means were significantly different, with the DFI score increasing with severity (p><0.0001). The DFI score did not differ across occupation (n>=0.561), marital status (p=0.428) or primary caregiver (Λ=0.679). There was no significant correlation between the DFI score and the number of dependants (r=0.147, p=0.080).
The DFI score differed across treatment duration (p<0.001), with scores generally decreasing as treatment duration increased. The median (interquartile range) DFI score for newly diagnosed cases was 20 (13.5 - 23.5), 13.5 (9 - 18.5) for treatment <1 year and 8.5 (3 - 15) for treatment >1 year.
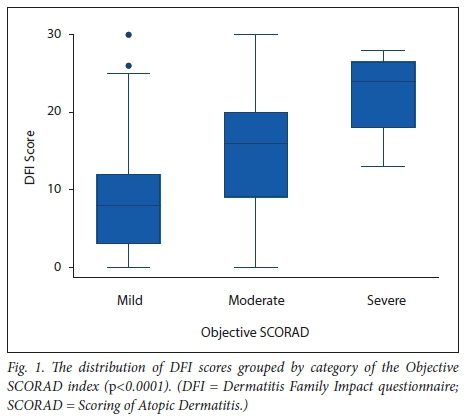
Table 2 shows the DFI responses for categories of the Objective SCORAD index.
Housework
Caring for a child with AD had little effect on activities such as washing and cleaning in 35% (n=50) of all cases. 'No effect' or 'a little' was noted in 32% (n=20) and 34% (n=21) of mild cases of AD, respectively. Housework was affected 'a lot' or 'very much' in 50% (n=38) of moderate cases.
Food preparation and feeding
This category referred to purchasing of specific foods or ingredients, dietary restrictions of the child with AD and family members, extra time and attention needed for meal preparation and the costs involved. 'Little' or 'no effect' was reported in 76% (n=47) of mild cases, whereas the effect was reported as 'a lot' in 32% (n=24) of moderate cases.
Sleep
In moderate cases, 32% (n=24) of caregivers described their sleep as being affected 'a lot' and 26% (n=20) claimed the effect was 'very much'. Sleep was not affected at all in 48% (n=30) of mild cases.
Family leisure activities, shopping and expenditure
Activities such as swimming were not affected in 51% (n=73) of patients. Only 28% (n=40) of caregivers reported that caring for a child with AD had 'a lot' or 'very much' of an effect on all aspects of shopping for their family. Costs related to treatment, clothing, etc. were 'a lot' in 32% (n=46) of all cases.
Tiredness and emotional distress of caregivers
Responses to questions on tiredness had a fair representation, with 29% (n=41) of caregivers reporting no tiredness caused by caring for a child with AD, 27% (n=39) describing the effect as 'a little' and 27% (n=38) as 'a lot. Feelings such as depression, frustration or guilt were described by caregivers in 27% (n=39) of all cases. 'A lot' of emotional distress was noted in 36% (n=27) of moderate cases, whereas this was expressed in only 19% (n=12) of mild cases.
Relationships
The relationship of the caregiver with their partner and other children was affected 'a lot' in 32% (n=24) of moderate cases and in 19% (n=12) of mild cases.
Helping with treatment
Helping with the child's treatment affected the caregiver's life in 36% (n=51) of all AD cases. The effect was experienced as 'a lot' in 42% (n=32) of moderate cases. Caregivers reported 'no effect' in 39% (n=24) of mild cases.
Correlation between the Objective SCORAD index and the Dermatitis Family Impact questionnaire
The overall responses to the DFI questions were significantly associated (p <0.05) with the Objective SCORAD index, except for the categories 'housework' and 'expenditure' (p=0.056 and £=0.153, respectively). QOL factors significantly affected were emotional distress of the caregiver (p<0.0001), tiredness of the caregiver (p<0.0001) and family leisure activities (p<0.0001). Helping with treatment (p=0.016), food preparation and feeding (p=0.003), sleep of family members (p=0.001) and the caregiver's relationships (p =0.025) were also moderately affected. Fig. 1 shows the distribution of DFI scores for each category of the Objective SCORAD index.
Discussion
Our study demonstrates that in the SA context, the QOL of caregivers of children with AD is adversely affected. In addition, a significant correlation between disease severity and QOL was demonstrated, i.e. an increase in severity of AD was associated with a decline in QOL.
Most participants were black, which reflects the predominant demographic in our setting. Owing to the study being conducted at a tertiary-level hospital, partiality to increased disease severity was anticipated; however, severe cases were limited. Moderate and mild AD cases were well represented.
It is expected that younger children depend more on their carers for all aspects of daily living. The mean (SD) age of patients was 5 (4) years, suggestive of such demand. The main caregiver in our study was a mother, father or grandmother.
In AD, practical care issues such as extra cleaning or laundry due to soiled clothes from weeping skin or greasy ointments can cause increased stress in the caregiver's life.[15] In our study, the correlation between housework and disease severity (p=0.056) did not reach statistical significance. Although our interview guide included questions about dependants and partners, it did not include questions regarding other family members or housekeepers at home who may have assisted with housework.
QOL improved with longer treatment duration, as evidenced by a lower DFI score. This may be due to AD improving with treatment and also caregivers' greater understanding and management of the disease.
There is no clear evidence for the role of dietary restrictions in the control and prevention of AD.[16,17] However, many carers do believe that AD is caused by something in the diet.[9,16] This opinion often leads to dietary restrictions and separate diets for the patient and their family, which increase the burden of caring for a child with AD and introduce unnecessary costs. If a patient has had appropriate and adequate topical treatment and still has moderate to severe eczema, a concomitant food allergy should be investigated.[16] Educating caregivers about these aspects is essential in managing and alleviating the burden of disease. We noted that no difficulty in food preparation and feeding was associated with mild AD.
In our study, more than half of moderate cases' caregivers reported a significant effect on sleep. Chamlin et al[19]noted that in an attempt to prevent scratching and awakening, parents may opt for co-sleeping as a strategy to improve the child's sleep. This practice may lead to disrupted sleep patterns in parents and may be detrimental to the child owing to the risk of habitual behaviour even when the disease is controlled. Moore et al.[18]calculated that parents spend an average of 45 minutes per night with a child with eczema and that the extent of the disruption increases with severity of the disease. This, coupled with the extra attention needed to care for a child with AD, leads to fatigue or exhaustion. In SA, many families may have limited sleeping facilities and share beds. This and other domestic aspects such as ablution facilities and how they may influence caring for an AD patient were not evaluated in this study, but should be explored further.
The effect on family leisure activities such as swimming and holidaying showed a positive correlation with AD severity. Reljic et ali19 observed that parents of children with AD restricted their children from swimming in a pool, presumably to avoid exacerbation of AD by chlorine oxidants. In addition, AD patients may participate less in leisure activities owing to fatigue, disease severity and stigmatisation.[19] According to Fourie et al.,[20] social, cultural and individual contexts in which adolescents live have an influence on how leisure time is spent. In SA, a lack of finances and resources and also safety concerns are some of the factors that influence and restrict recreation[21]
Another contributor to increased family stress is the financial cost of caring for a child with AD, which relates to disease severity.[10] This more commonly affects those living in low-income households. Su et al[22]found that caring for a child with moderate or severe AD had a higher financial burden than caring for one with insulin-dependent diabetes mellitus. Although caregivers generally found the costs related to treatment, clothing, etc. to be 'a lot', there was, unexpectedly, no significant association with AD severity when correlated with the Objective SCORAD index. Most caregivers had a stable source of income, such as full-time employment, income from the other parent or a child support grant. In addition, free medication from primary healthcare facilities possibly alleviates the financial burden of caring for a child with AD.
Caregivers often report having limited support from their social network with regard to others' willingness to care for their sick child.
In a study by Chamlin et al.,[19]parents described untoward reactions such as accusations of abuse or neglect from extended family and strangers. Such reactions and limited support were attributed to having a child with AD and became a constant reminder of the disease and a source of guilt and frustration. In addition, the stigma associated with the child's appearance and the fear of contagion result in social isolation. Parents with a history of atopy themselves may feel personally responsible for their child's condition and experience guilt or self-blame. We noted that such feelings were reported particularly in severe and moderate cases of AD.
With regard to marital status, 'single' referred to a caregiver not having a partner and therefore providing solely for the child with AD. The description 'unmarried' referred to the caregiver having a partner, whether cohabiting or not. This distinction was explained to caregivers. It was postulated to be an important contributor to financial and emotional support and established caregivers' current relationships.
Most caregivers had a partner and, on average, one other dependant younger than 16 years. The effect of AD on these relationships correlated significantly with disease severity. Having additional dependants did not affect caregivers' QOL.
The relationship between the caregiver and the child with AD can also be affected. Discipline has been identified as an area in which caregivers function suboptimally.[23] Parents often submit to or overindulge the child to avoid conflict and worsening their distress. Daud et al.[24]reported the most striking behavioural disturbances in children with AD as clinginess, dependency and attention-seeking, which may affect relationships with other family members. A detailed survey of these characteristics in children with AD was not included in the current study, but is recommended for future studies.
Caregivers may have worries relating to medication use, particularly topical steroids. Anxiety regarding their side-effects often leads to a steroid phobia.PJ1 Lack of knowledge is thought to be a significant contributing factor to poor treatment adherence and treatment failure.[22] In our study, being involved in the treatment of a child with AD had a significant effect on caregivers' QOL in most cases. The majority of patients had no comorbidities, which suggests that patient care focused on AD and was not affected by other medical conditions.
Unfortunately, severe cases of AD were not representative in our study. The response trend was either 'a lot' or 'very much', which made analysis of these results challenging. Future studies of a larger sample size and comparable representation across all grades of AD severity are needed to substantiate an association between QOL factors and AD.
Housework and expenditure did not correlate with disease severity in our study. Although housework and expenditure were not necessarily increased, caring for a child with AD may still have affected these factors to some extent. Owing to context-specific sociodemographic and -economic factors in developing countries such as SA, AD may not receive the same attention as in developed countries and hence financial implications may not be as severely felt. There are currently no SA studies on the costs of childhood AD. This is an important subject for future studies.
As our study population was drawn from a single-hospital, tertiary-level care department, it was not representative of the overall population and therefore limits the generalisability of the findings.
Conclusion
In the SA context, caring for a child with AD adversely affects caregivers' QOL, which further declines as disease severity increases. Factors such as emotional wellbeing, tiredness and emotional distress of the caregiver, family relationships, leisure activities and being involved with treatment are particularly affected.
In addition to a lack of understanding of the burden of the disease on caregivers in the SA context, the existing system for managing AD is not equipped to support caregivers' emotional and social needs. The unique sociodemographic and -economic factors in developing countries such as SA warrant an assessment of factors that particularly affect AD in this setting to offer patients more holistic care. Although compiling detailed histories that focus on psychosocial aspects can be time consuming, such a study is necessary to comprehensively evaluate and manage the psychosocial aspects affecting the patient's caregiver and their family life. Such an approach could contribute to optimal management of the patient and the development of practical interventions to improve AD outcome.
Declaration. This manuscript was submitted in partial fulfilment of the requirements for the degree of Master of Medicine.
Acknowledgements. We thank the staff of the dermatology clinic at Ore1 Hospital for their valuable assistance in this study and the patients and caregivers for their participation.
Author contributions. AM conceptualised the study. BS was responsiblefor writing the protocol (guided by AM), obtaining research approval an data collection and, together with YT and YB, data analysis. YT and AM contributed to interpretation of results. BS was the primary author and was assisted by YT and YB in preparing the manuscript.
Funding. None.
Conflicts of interest. None.
References
1. Simpson EL, Hanifin JM. Atopic dermatitis. J Am Acad Dermatol 2005;53:115- 128. https://doi.org/10.1016%2Fj.jaad.2005.01.130 [ Links ]
2. Todd O. Epidemiology of atopic dermatitis. S Afr Med J 2014;104(10):710. https://doi.org/10.7196/samj.8843 [ Links ]
3. Manjra AI, Du Plessis P, Weiss R, et al. Childhood atopic eczema consensus document. S Afr Med J 2005;95(6 Part 2):435-440. [ Links ]
4. Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phase One and Three repeat multicountry cross-sectional surveys. Lancet 2006;368:733-743. https://doi.org/10.1016/s0140-6736(06)69283-0 [ Links ]
5. Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol 2009;124:1251-1258. https://doi.org/10.1016/j.jaci.2009.10.009 [ Links ]
6. Todd O, Saxe N, Milne J, Tolosana S, Williams H. Prevalence of atopic dermatitis in Xhosa children living in rural, peri-urban, and urban areas. Curr Allergy Clin Immunol 2004;17:140. [ Links ]
7. Lewis-Jones S. Quality of life and childhood atopic dermatitis: The misery of living with childhood eczema. Int J Clin Pract 2006;60:984-992. https://doi.org/10.1111%2Fj.1742-1241.2006.01047.x [ Links ]
8. Warschburger P, Buchholz HT, Petermann F. Psychological adjustment in parents of young children with atopic dermatitis: Which factors predict parental quality of life? Br J Dermatol 2004;150(2):304-311. https://doi.org/10.1111/j.1365-2133.2004.05743.x [ Links ]
9. Chamlin SL, Frieden IJ, Williams ML, Chren MM. Effects of atopic dermatitis on young American children and their families. Pediatrics 2004;114(3):607-611. https://doi.org/10.1542/peds.2004-0374 [ Links ]
10. Kemp AS. Atopic eczema: Its social and financial costs. J Paediatr Child Health 1999;35:229-231. https://doi.org/10.1046/j.1440-17541999.00343.x [ Links ]
11. Al Shobali HA. The impact of childhood atopic dermatitis on the patients' family. Pediatr Dermatol 2010;27(6):618-623. https://doi.org/10.1111/j.1525-1470.2010.01215.x [ Links ]
12. Williams HC, Burney POJ, Pembroke AC, Hay RJ. The U.K. Working Party's Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol 1994;131(3):406-416. https://doi.org/10.1111/j.1365-2133.1994.tb08532.x [ Links ]
13. Oranje AP, Olazenburg EJ, Wolkerstorfer A, De Waard-Van der Spek FB. Practical issues on the interpretation of scoring atopic dermatitis: The SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol 2007;157(4):645-648. https://doi.org/10.1111/j.1365-2133.2007.08112.x [ Links ]
14. Dodington SR, Basra MK, Finlay AY, Salek MS. The Dermatitis Family Impact questionnaire: A review of its measurement properties and clinical application. Br J Dermatol 2013;169(1):31-46. [ Links ]
15. Lawson V, Lewis-Jones MS, Finlay AY, Reid P, Owens RO. The family impact of childhood atopic dermatitis: The Dermatitis Family Impact questionnaire. Br J Dermatol 1998;138(1):107-113. https://doi.org/10.1046/j.1365-2133.1998.02034.x [ Links ]
16. Jordaan HF, Todd O, Sinclair W, Oreen RJ. Aetiopathogenesis of atopic dermatitis. S Afr Med J 2014;104(10):706-709. https://doi.org/10.7196/samj.8840 [ Links ]
17. Bath-Hextall F, Delamere FM, Williams HC. Dietary exclusions for improved established atopic dermatitis in adults and children: Systematic review. Allergy 2009;64:258-264. https://doi.org/10.1111/j.1398-9995.2008.01917.x [ Links ]
18. Moore K, David TJ, Murray CS, Child F, Arkwright PD. Effect of childhood eczema and asthma on parental sleep and well-being: A prospective comparative study. Br J Dermatol 2006;154(3):514-518. https://doi.org/10.1111/j.1365-2133.2005.07082.x [ Links ]
19. Reljic V, Oazibara T, Nikolic M, Zaric M, Maksimovic N. Parental knowledge, attitude, and behavior toward children with atopic dermatitis. Int J Dermatol 2017;56(3):314-323. https://doi.org/10.1111/ijd.13529 [ Links ]
20. Fourie J, Slabbert E, Saayman M. The leisure and sport participation patterns of high school learners in Potchefstroom. S Afr J Res Sport Phys Educ Recreat 2011;33(1):65-80. https://doi.org/10.4314/sajrs.v33i1.65488 [ Links ]
21. Wegner L. Through the lens of a peer: Understanding leisure boredom and risk behaviour in adolescence. S Afr J Occup Ther 2011;41(1):18-24. [ Links ]
22. Su JC, Kemp AS, Varigos OA, Nolan TM. Atopic eczema: Its impact on the family and financial cost. Arch Dis Child 1997;76(2):159-162. https://doi.org/10.1136/adc.76.2.159 [ Links ]
23. Oundersen C, Mahatmya D, Oarasky S, Lohman B. Linking psychosocial stressors and childhood obesity. Obes Rev 2011;12(5):e54-e63. https://doi.org/10.1111/j.1467-789x.2010.00813.x [ Links ]
24. Daud LR, Oarralda ME, David TJ. Psychosocial adjustment in preschool children with atopic eczema. Arch Dis Child 1993;69(6):670-676. https://doi.org/10.1136/adc.69.6.670 [ Links ]
 Correspondence:
Correspondence:
B Singh
bhavnasingh70@gmail.com
Accepted 23 August 2018.














