Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.13 no.1 Pretoria Abr. 2019
http://dx.doi.org/10.7196/sajch.2019.v13i1.1564
ARTICLE
Severe hypertension in children at a central referral hospital in KwaZulu-Natal Province, South Africa
D Murigo-ShumbaI; R BhimmaII; E NaickerIII
IMB ChB, DCH, Dip HIV Man (SA); Department of Maternal and Child Health, Nelson R Mandela School of Medicine, College of Health Sciences, University of KwaZulu-Natal Durban, South Africa
IIMB ChB, DCH, FCP (Paeds), MMed (Paed), MD, Cert Nephrology (Paed); Department of Maternal and Child Health, Nelson R Mandela School of Medicine, College of Health Sciences, University of KwaZulu-Natal Durban, South Africa
IIIMB ChB, DCH, FCP (Paed), Cert Nephrology (Paed); Department of Maternal and Child Health, Nelson R Mandela School of Medicine, College of Health Sciences, University of KwaZulu-Natal Durban, South Africa
ABSTRACT
BACKGROUND: Hypertension (HPT) is often underdiagnosed in children, although significant morbidity and mortality arises from hypertensive target organ damage and hypertensive crises
OBJECTIVES: To determine the prevalence, complications and causes of severe HPT in children <12 years old at a central hospital
METHODS: Hospital records of children < 12 years old with severe HPT (stage 2 and higher) from 2005 to 2014 were reviewed. Demographics, nutritional status, causes, HIV status, presence of target organ damage and treatment were analysed
RESULTS: Of 821 children admitted to the paediatric nephrology unit, 152 (18.5%) children had severe HPT, with a mean age of 6.3 years; 86 (57%) were boys. A total of 28 (19%) were HIV-positive, and 19 (68%) were treatment naive. Kidney disease accounted for 82% of cases, 46 (30%) having steroid-resistant nephrotic syndrome, 22 (14%) HIV-associated nephropathy, 19 (13%) glomerulonephritis, 21 (14%) congenital urinary tract abnormalities and 17 (11%) other renal causes. Renovascular causes accounted for 12 (8%) cases. Of these 12,7 (58%) had left ventricular hypertrophy (LVH), compared with 10/125 (8%) who had other forms of kidney disease (jkO.023). Hypertensive crises occurred in 28 (18%) patients, and were significantly more common in children with HPT secondary to renovascular causes than renal causes (p=0.001
CONCLUSION: Renal diseases were the most common cause of severe HPT in children. Hypertensive crises, retinopathy and LVH are common in renovascular HPT
Hypertension (HPT) is a major non-communicable disease affecting more than one billion people worldwide, with a rising prevalence. A study that estimated worldwide blood pressure (BP) trends from 1975 to 2015 showed an increase in adults with high BP from 594 million in 1975 to 1.13 billion in 2015, with the highest prevalence noted in low-income countries.[1] The morbidity and mortality associated with HPT is increasing, as revealed in a study that showed a rise in disability-adjusted life years between 1990 and 2015.[2] There is evidence from many studies of BP tracking from childhood to adulthood.[3,4] It is therefore important to detect and treat HPT early so as to reduce the risk of complications.
HPT in children is underdiagnosed as there is often no measurement of BP in children presenting to healthcare facilities,[3] and at times incorrect measuring techniques are used. The prevalence of HPT may therefore be an underestimate, as some cases may have been missed. In a systemic review and meta-analysis of 25 African studies that recruited patients from 1997 to 2013, the pooled prevalence of HPT was found to be 5.5% and that of pre-HPT 12.7% among children and adolescents.[5] A local study in school-age children in the Phoenix area in Durban, South Africa (SA), revealed a prevalence of 2.1% for pre-HPT and 2.6% for HPT.[6] Another school survey by the National Kidney Foundation of SA (NKFSA) over a 9 - 10-year period showed hypertension in 12% of female and 16% of male adolescents.[7] Other SA studies reported HPT prevalence ranging from 1 to 24.4% in children.[3,8-11]
In younger children, secondary HPT is more common than primary with renal and renovascular causes making up the largest proportion. The prevalence of primary HPT has, however, been increasing in older children owing to sedentary lifestyles that result in obesity.[12-14]
Hypertensive children should be assessed for target organ damage, which can affect the heart, retina, kidneys and nervous system.[12]
Hypertensive target organ damage is an indication for starting antihypertensive medication.[15] Left ventricular hypertrophy (LVH) is the most common form of target organ damage seen in children.[15] It is a risk factor for future cardiac disease, and treatment results in its regression and reduction in cardiac risk.[16]
While in adults severe HPT can be defined as systolic BP >160 mmHg, or diastolic BP >100 mmHg, in children it has not been clearly defined. In one review on severe HPT in children and adolescents, severe HPT was defined as stage 2 HPT with severe symptoms,[17] while in another study, it was defined as BP above stage 2 HPT with associated symptoms. [18] Severe HPT can be divided into hypertensive emergency and hypertensive urgency. Hypertensive emergency is severe HPT with target organ damage and life-threatening symptoms such as seizures and encephalopathy, while hypertensive urgency has less serious symptoms such as vomiting and headache, with no target organ damage. The management of these two forms of hypertensive crisis differ as there is a more urgent need to reduce the BP in hypertensive emergency.[15,17]
The present study aimed to determine the prevalence of severe HPT, secondary causes, presence of target organ damage and the incidence and treatment of hypertensive crises among patients admitted to the paediatric nephrology unit at Inkosi Albert Luthuli Central Hospital (IALCH), Durban, SA.
Methods
This 10-year retrospective chart review was done at IALCH, which serves as a quaternary referral hospital for KwaZulu-Natal and its neighbouring provinces. Computerised hospital records of patients admitted to the paediatric nephrology unit at IALCH from 2005 to 2014 were reviewed. Inclusion criteria were all children <12 years with severe HPT. Severe HPT was defined as stage 2 HPT as defined by the 'Fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents',[15] which classifies BP as follows: normal if <90th percentile, pre-HPT if >90th to <95th percentile or > 120/80 mmHg in adolescents; stage 1 HPT if >95th to 99th percentile plus 5 mmHg, stage 2 HPT if >99th percentile plus 5 mmHg. BP was measured using automatic non-invasive BP devices with an appropriately sized cuff. Blood pressure was measured three times to confirm stage 2 HPT. However, for patients who had signs and symptoms of hypertensive crisis, one reading in the stage 2 HPT category was adequate to classify the patient as stage 2. A Pediatric BP Calculator (PedsBP, Shuojing Song, USA) and/or age- and sex-specific BP tables with height percentiles that utilised data from the above report were used to classify HPT. Patients excluded from the study were those >12 years old, children with HPT less than stage 2 and those managed under other specialist units.
Data collected included demographic data, nutritional status, causes of HPT, HIV status, presence of target organ damage and hypertensive crisis and its treatment. Nutritional status was assessed using World Health Organization Child Growth Standards.[19-21] Children were classified using BMI or weight-for-length/height: wasted if <-2; normal if -2 to +2; overweight if >+2 to +3; and obese if >+3.
Target organ damage assessed for included LVH as determined by echocardiography undertaken by a paediatric cardiologist, nephropathy based on presence or absence of proteinuria on urine dipstick analysis, retinopathy and cerebrovascular accidents. Hypertensive crisis information included the number of crises, and the symptoms and treatment of each. Information was captured in Excel (Microsoft, USA) and analysed by a professional statistician from the School of Public Health, Biostatistics Department, University of KwaZulu-Natal.
Permission to conduct the study was obtained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (ref no. BE507/15).
Results
One hundred and fifty-two (18.5%) of 821 children admitted over the 10-year period had severe HPT, as shown in Fig. 1. Table 1 shows the number of patients in each age group. The mean age of presentation was 6.3 years (range 10 days - 12 years). A total of 86 (57%) were male and 66 (43%) female. Race and nutritional status are shown in Table 2. Twenty-eight (19%) children were HIV-positive; of these, 19 (68%) were not on antiretroviral treatment on presentation (Table 2).

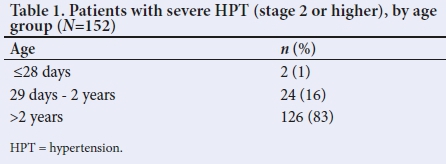

Table 3 shows the causes of severe HPT in the study group. Renal causes accounted for 82% of cases, the most common cause being nephrotic syndrome. Renovascular causes accounted for 12 (8%) cases, Takayasu arteritis 8 (5%) and renal artery stenosis 4 (3%). Systemic causes were noted in 3 (2%) children. All patients with severe HPT secondary to steroid-resistant nephrotic syndrome received prednisolone in an attempt to induce remission. However, severe HPT was only diagnosed when patients were on low-dose steroids (<0.5 mg/kg/day) or weaned off steroids, to remove steroids as a possible causal agent for severe HPT.

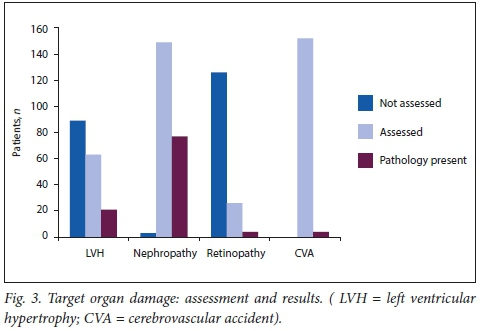
Sixty-three (42%) children were assessed for LVH, of whom 21 (33%) were found to have this. Ten (48%) children with LVH had renal causes, while 7 (33%) had renovascular causes. However, LVH affected patients with renovascular causes more often than patients with renal causes, as 58% (7/12) with renovascular causes had LVH, compared with 8% (10/125) who had other forms of kidney disease (p<0.023). Although this difference was statistically significant, the proportion of patients not assessed for LVH among those with renal causes was high, as only 33% of patients with renal causes were assessed for LVH.
Seventy-seven (51%) children had nephropathy. Of these, 70 (91%) had severe HPT secondary to renal aetiology, 4 (5%) renovascular aetiology and in 3 (4%), the aetiology was unknown. Four (3%) had hypertensive retinopathy. Cerebrovascular accident (CVA) was noted in 4 (3%) patients. Retinopathy and CVA were noted more frequently in those with renovascular causes (75%) as opposed to renal causes (25%), but this was not statistically significant (p>0.3).
Hypertensive crises occurred in 28 (18%) patients. Of these, 22 (79%) had 1 hypertensive crisis, 4 (14%) had 2 and 2 (7%) had 3 crises. The most common symptoms noted were encephalopathy and seizures, which occurred in 25 (89%) and 24 (86%) children, respectively (Fig. 2). The most commonly used drugs for the crises were nifedipine and labetalol, which were used as the sole agent in 11 (40%) and 4 (14%) children, respectively. Four (14%) children had a combination of both drugs used sequentially. Less frequently used drugs included sodium nitroprusside, furosemide and amlodipine. Seventeen (61%) children with hypertensive crises were treated with 1 drug only, while 5 (18%) were treated with 2 drugs. Hypertensive crisis was significantly more common among the children with renovascular causes than in those with renal causes (OR 7.5; 95% CI 2.2 - 26.2; p=0.001) of HPT.

Discussion
There was an 18.5% prevalence of severe HPT (stage 2) among children admitted to the paediatric nephrology unit at IALCH over the 10-year study period. While most previous studies determined the prevalence of HPT in children, there is a limited number of studies that assessed specifically for severe HPT in children. In keeping with reports from other studies of HPT in children, males in the present study were more commonly affected by severe HPT than females, at 57% and 43%, respectively, a ratio of almost 3:2. In another study on HPT prevalence, cardiac complications and antihypertensive medication use in children, 61% of patients were male and 39% female.[22]
Antiretroviral treatment for HIV-positive patients reduces the incidence of HIV-associated nephropathy (HIVAN). In this study, 14% of the patients with severe HPT had HIVAN, making it the second most common cause of severe HPT after nephrotic syndrome. Free antiretroviral treatment has been offered in the public sector in SA since April 2004, but despite this favourable development, 19 (68%) HIV-positive children were not on treatment.
The majority of the children had severe HPT due to secondary causes, and 12 (8%) children had HPT of unknown aetiology despite extensive investigations. In older children and adolescents, primary HPT is more common than secondary, which is more often seen in younger children.[12,23] In a study by Gupta-Malhotra et αl.[24] on essential (primary) v. secondary HPT that included children from birth to 19 years, essential HPT occurred at a significantly older age than secondary HPT, with median ages at diagnosis of 12 years (range 3-17 years) and 9 years (range 0.08 - 19 years), respectively.The lower prevalence of primary HPT in the current study may be due to the exclusion of children older than 12 years, and of those with less severe forms of HPT, since primary HPT presents in less severe forms.
The majority of HPT patients in the current study ( 125; 82%) had a renal aetiology. This is in keeping with the results of other studies. A study in Romania looking at aetiology and BP patterns in secondary HPT in children showed that renal parenchymal disease accounted for 83.2% of secondary HPT, which is similar to the present study's results.[25] Two other studies that have demonstrated renal causes as the most common causes of secondary HPT are a Polish[26] and a Thai[27] study in which 68% and 62.7%, respectively, of their cases of HPT were due to renal parenchymal disease. In the present study, renovascular causes accounted for 8% of the cases of severe HPT, which is similar to the Polish study above in which renovascular causes accounted for 10% of the cases.[26]
While CVA and hypertensive nephropathy were assessed in the present study in almost all patients with severe HPT, LVH was only assessed in 42% of patients, and even fewer were assessed for hypertensive retinopathy (17%), as shown in Fig. 3. This was a result of limited resources, and staffing constraints.
It is recommended by the 'Fourth report on the diagnosis, evaluation and treatment of high blood pressure in children and adolescents that all children and adolescents with established HPT be assessed by echocardiogram for LVH.[15] Regression of LVH has been noted with antihypertensive treatment. A study by Kupferman et al.[28] showed that there may be a decline in LVH with BP reduction in children with chronic kidney disease. In the present study, only 42% of patients were assessed for LVH, owing to limited resources and staffing constraints. Of these, 33% were noted to have LVH. In a US study by Hanevold et αl.,[29] the prevalence of LVH was 41.1%.[29] The proportion of patients with LVH in the present study was lower than this, possibly because fewer patients were assessed for LVH.
Proteinuria as a marker of hypertensive nephropathy was difficult to assess in this study, as the majority of patients had nephrotic syndrome, meaning that the presence of proteinuria was more likely due to the primary disease than it was a complication of HPT.
Hypertensive retinopathy is well documented in adults; however there are limited studies available on children. In a study by Williams et al.[30]that assessed hypertensive retinopathy in severely hypertensive children, 18% of the children who had eye assessments had hypertensive retinopathy. The same study showed that in patients with renovascular disease, there was a higher prevalence of hypertensive retinopathy, although this was not statistically significant.[30] In our study, 4 (15%) patients had hypertensive retinopathy, comparable to the results of the study by Williams et al.[30]with 3 (75%) having HPT secondary to renovascular causes.
HPT is a known risk factor for CVA in adults, and improved BP control reduces this risk. In children, HPT may be an important factor in the development of stroke.[31] In our study, 4 (3%) patients had CVAs, with 3 (75%) of these having HPT secondary to renovascular causes.
Hypertensive crisis occurred in 28 (18%) children. The most common presentations in the present study were encephalopathy and seizures, which occurred in 89% and 85% of the cases, respectively Unlike these results, a study in Taiwan showed headache (54.5%) and dizziness (45.5%) as the two most common presenting symptoms.[32] In the present study, the proportion of patients with hypertensive crisis was significantly higher among children with renovascular causes of HPT than intrinsic renal causes (58% v. 16%, p<0.001).
Intravenous antihypertensive therapy is recommended for hypertensive emergencies for a controlled reduction in BP,[15] aiming to reduce it by 25% in the first 8 hours, then gradually over 24 - 48 hours. Hypertensive urgencies can be treated with oral or intravenous antihypertensives.[15] The recommended intravenous drugs include esmolol, hydralazine, labetalol, nicardipine and sodium nitroprusside.[15] In the present study, almost 90% of patients with hypertensive crisis had hypertensive emergencies, yet only 36% were treated with intravenous antihypertensives. The most commonly used antihypertensive was oral nifedipine. The reason for the limited use of intravenous antihypertensives is that the majority of patients were treated in a general ward, owing to limited space in the intensive care unit. In the general ward, close intensive BP monitoring is not possible, owing to limited staff and equipment, hence the use of oral nifedipine, which is also more readily available and is more easily administered in a general care setting.
The strengths of the current study are that it was carried out over a long period of time and a significantly large number of patients were included in the study. A limitation of the study is that it was conducted at a single centre and not all parameters, such as LVH and retinopathy, were assessed in every patient (Fig. 3). This limits the conclusions that can be drawn from some of the data obtained. Another limitation of the study is that, being retrospective, the actual technique of BP measurement in the children may not have been standardised over the period.
Conclusion
Our findings of severe HPT in children aged <12 years showed that it occurred more commonly in males. The most common cause was glomerular disease, with the majority of the patients having steroid-resistant nephrotic syndrome. Renovascular HPT is the more severe form, as a higher proportion of these patients had target organ damage and hypertensive crises. A high proportion of children with HIV infection who were not on antiretroviral treatment were diagnosed with severe HPT
Based on our current findings, we strongly recommended HIV testing in all children, with early commencement of treatment in those who test HIV-positive, to reduce the prevalence of HIVAN, which is a significant cause of severe HPT. Long-term studies should be done in our population to monitor the progression of LVH and retinopathy in children on antihypertensive treatment. Children presenting with encephalopathy or seizures should have their BP checked as these were the most common presentations in children with hypertensive crisis.
Acknowledgements. We would like to thank the medical manager of the IALCH for permission to publish this data, the staff involved in the care of these children and those at the hospitals that referred these children to our centre.
Author contributions. DM-S: data capture, analysis and writing of the manuscript. RBh: conceptualisation of the project, data analysis and review of the manuscript. EN: patient care, data capture and review of
the manuscript.
Funding. None.
Conflict of Interest. None.
References
1. NCD Risk Factor Collaboration. Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017;389(10064):37-55. https://doi.org/10.1016/S0140-6736(16)31919-5 [ Links ]
2. Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mmHg, 1990 - 2015. JAMA 2017;317(2):165-182. https://doi.org/10.1001/jama.2016.19043 [ Links ]
3. Kagura J, Adair LS, Musa MG, Pettifor JM, Norris SA. Blood pressure tracking in urban black South African children: Birth to twenty cohort. BMC Pediatr 2015;15(1):78. https://doi.org/10.1186/sl2887-015-0402-z [ Links ]
4. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review and meta-regression analysis. Circulation 2008; 117(25) :3171-3180. https://doi.org/10.1161/circulationaha.107.730366 [ Links ]
5. Noubiap JJ, Essouma M, Bigna JJ, Jingi AM, Aminde LN, Nansseu JR. Prevalence of elevated blood pressure in children and adolescents in Africa: A systematic review and meta-analysis. Lancet Pub Health 2017;2(8):e375-e386. https://doi.org/10.1016/s2468-2667(17)30123-8 [ Links ]
6. Bhimma R, Pillay M, Naidoo D, Vanden Burg MF, Smuts P, Onia R. Hypertension prevalence and risk factors in children and adolescents of school-going age in the Phoenix Area, Durban, South Africa. Pediatr Nephrol 2014;29:2434. [ Links ]
7. Meyers AM. Promotion of kidney care in countries with limited resources: How does the National Kidney Foundation of South Africa fare? Clin Nephrol 2016;86(Suppl 1):S69-S73. https://doi.org/10.5414/cnp86s111 [ Links ]
8. Monyeki KD, Kemper HCG, Makgae PJ. The association of fat patterning with blood pressure in rural South African children: The Ellisras Longitudinal Growth and Health Study. Int J Epidemiol 2006;35(1):114-120. https://doi.org/10.1093/ije/dyi219 [ Links ]
9. Nkeh-Chungag BN, Sekokotla AM, Sewani-Rusike C, Namugowa A, Iputo JE. Prevalence of hypertension and pre-hypertension in 13-17 year old adolescents living in Mthatha - South Africa: A cross-sectional study. Cent Eur J Public Health 2015;23(l):59-64. https://doi.org/10.21101/cejph.a3922 [ Links ]
10. Moselakgomo VK, Toriola AL, Shaw BS, Goon DT, Akinyemi O. [Body mass index, overweight, and blood pressure among adolescent schoolchildren in Limpopo province, South Africa.] Rev Paul Pediatr 2012;30(4):562-569. https://doi.org/10.1590/S0103-05822012000400015 [ Links ]
11. Schutte AE, Van Rooyen JM, Huisman HW, Kruger HS, Malan NT, de Ridder JH . Dietary risk markers that contribute to the aetiology of hypertension in black South African children: The THUSA ΒΑΝΑ study. J Hum Hypertens 2013:17(1):29-35. https://doi.org/10.1038/sj.jhh.1001508 [ Links ]
12. Spagnolo A, Giussani M, Ambruzzi AM, et al. Focus on prevention, diagnosis and treatment of hypertension in children and adolescents. Ital J Pediatr 2013;39(1):20. https://doi.org/10.1186/1824-7288-39-20 [ Links ]
13. Sorof JM, Lai D, Turner J, Poffenbarger Τ, Portman RJ. Overweight, ethnicity and the prevalence of hypertension in school-aged children. Pediatrics 2004;113(3):475-482. https://doi.org/10.1542/peds.113.3.475 [ Links ]
14. Malatesta-Muncher R, Mitsnefes MM. Management of blood pressure in children. Curr Opin Nephrol Hypertens 2012;21(3):318-322. https://doi.org/10.1097/MNH.0b013e328351c415 [ Links ]
15. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. National Institute of Health publication 05:5267. Bethesda: National Heart, Lung, and Blood Institute, 2005. [ Links ]
16. Devereux RB, Dahlo'f B, Gerdts Ε, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: The Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation 2004;110(11):1456-1462. https://doi.org/10.1161/01.cir.0000141573.44737.5a [ Links ]
17. Flynn JT, Tullus, K. Severe hypertension in children and adolescents: Pathophysiology and treatment. Pediatr Nephrol 200924(6):1101-1112. https://doi.org/10.1007/s00467-008-1000-l [ Links ]
18. Flynn JT, Bradford MC, Harvey EM. Intravenous hydralazine in hospitalised children and adolescents with hypertension. J Pediatr 2015;168:88-92. https://doi.org/10.1016/j.jpeds.2015.07.070 [ Links ]
19. World Health Organization. The WHO Child Growth Standards. http://www.who.int/childgrowth/standards (accessed 18 January 2018). [ Links ]
20. World Health Organization. Growth reference 5-19 years, http://www.who.int/growthref/en (accessed 18 January 2018). [ Links ]
21. World Health Organization. Training Course on Child Growth Assessment. WHO Child Growth Standards. Interpreting Growth Indicators. http://www.who.int/nutrition/publications/childgrowthstandards_trainingcourse/en/ (accessed 18 January 2018). [ Links ]
22. Dobson CP, Eide M, Nylund CM. Hypertension prevalence, cardiac complications, and antihypertensive medication use in children. J Pediatr 2015;167(l):92-97. https://doi.org/10.1016/j.jpeds.2015.04.016 [ Links ]
23. Chandar J, Zilleruelo G. Hypertensive crisis in children. Pediatr Nephrol 2012;27(5):741-751. https://doi.org/10.1007/s00467-011-1964-0 [ Links ]
24. Gupta-MalhotraM, Banker A, Shete S, et al. Essential hypertension v. secondary hypertension among children. Am J Hypertens 2015;28(l):73-80. https://doi.org/10.1093/ajh/hpu083 [ Links ]
25. Gavrilovici C, Boiculese LV, Brumariu O, Dimitriu AG. Etiology and blood pressure patterns in secondary hypertension in children. Rev Med Chir Soc Med Nat Iasi 2007;111(1):70-81. [ Links ]
26. Wyszynska T, Cichocka E, Wieteska-Klimczak A, Jobs K, Januszewicz P. A single paediatric center experience with 1025 children with hypertension. Acta Paediatr 1992;81(3):244-246. https://doi.org/10.1111/j.1651-2227.1992.tb12213.x [ Links ]
27. Sumboonnanonda A, Chongcharoensuk C, Supavekin S, Pattaragarn A. Persistent hypertension in Thai children: Etiologies and outcome. J Med Assoc Thai 2006;89(Suppl 2):S28-S32. [ Links ]
28. Kupferman JC, Friedman LA, Cox C, et al. BP control and left ventricular hypertrophy regression in children with CKD. J Am Soc Nephrol 2014;25(1):167-174. https://doi.org/10.1681/asn.2012121197 [ Links ]
29. Hanevold C, Waller J, Daniels S, Portman R, Sorof J. The effects of obesity, gender, and ethnic group on left ventricular hypertophy and geometry in hypertensive children: A collaborative study of the International Pediatric Hypertension Association. Pediatrics 2004; 113(2):328-333. [ Links ]
30. Williams KM, Shan AN, Morrison D, Sinha MD. Hypertensive retinopathy in severely hypertensive children: Demographic, clinical and ophthalmoscopic findings from a 30-year British cohort. J Pediatr Ophthalmol Strabismus 2013;50(4):222-228. https://doi.org/10.3928/01913913-20130319-01 [ Links ]
31. Kupferman JC, Zafeiriou DI, Lande MB, Kirkham FJ, Pavlakis SG. Stroke and HPT in children and adolescents. J Child Neurol 2017;32(4):408-417. https://doi.org/10.1177/0883073816685240 [ Links ]
32. Yang W, Zhao L, Chen C, Wu Y, Chang Y, Wu H. First-attack paediatric hypertensive crisis presenting to the pediatric emergency department. BMC Pediatr 2012;12( 1):200. https://doi.org/10.1186/1471-2431-12-20C [ Links ]
 Correspondence:
Correspondence:
D Murigo-Shumba
davidzomurigo@gmail.com
Accepted 18 June 2018














