Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Child Health
versión On-line ISSN 1999-7671
versión impresa ISSN 1994-3032
S. Afr. j. child health vol.12 no.4 Pretoria dic. 2018
http://dx.doi.org/10.7196/sajch.2018.v12i4.1535
ARTICLE
Non-ketotic hyperglycaemia and the hemichorea–hemiballismus syndrome - a rare paediatric presentation
M P K HauptfleischI; J L RoddaII
IMB BCh, MMed Paeds, FCPaed (SA), Cert Paed Neuro (SA); Department of Paediatrics, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa and Chris Hani Baragwanath Academic Hospital, Johannesburg, Africa
IIMB BCh, FCPaed (SA) Department of Paediatrics, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa and Chris Hani Baragwanath Academic Hospital, Johannesburg, Africa
ABSTRACT
Hemichorea-hemiballismus may be due to non-ketotic hyperglycaemia, but this condition has rarely been described in paediatrics. We describe the case of a 13-year-old girl with newly diagnosed type 1 diabetes and acute onset of left-sided choreoathetoid movements. Neuroimaging revealed an area of hyperintensity in the right basal ganglia. Her blood glucose level at the time was 19 mmol/L, and there was no ketonuria. The hemiballismus improved with risperidone and glycaemic control. Repeat neuroimaging 4 months later showed complete resolution of the hyperintensities seen.
Non-ketotic hyperglycaemic hemichorea-hemiballismus (NKHHC) is a rare, reversible condition, with the clinical and radiological signs usually resolving within 6 months, following correction of hyperglycaemia.[1] The condition has previously been described as specifically affecting the elderly, with very few cases described in children and adolescents.[2]
We describe a paediatric patient presenting with the classic clinical and radiological findings of NKHHC.
Case
A 13-year-old female presented to hospital with severe cramps in her left hand. She reported that these had been of progressive severity over the past 10 days.
On examination she was noted to be dehydrated and was wasted (44 kg; 0.8 z-score). A blood gas done at the time of presentation showed: pH 7.36 BE -2.6 mmol/L; Ca 1.2 mmol/L (1.15 - 1.29); glucose 15.9 mmol/L (3.0 - 5.0).
A urine dipstick test was also performed and showed 4+ glucose; 1+ ketones.
She was diagnosed with type 1 diabetes mellitus, based on her age, and the rapidity of onset. This was subsequently confirmed with her testing positive for anti-glutamic acid decarboxylase and IA2 antibodies. She was assessed by the consultant paediatric endocrinologist, and admitted to start intravenous insulin therapy.
On the 5th day ofher hospital admission, she had two focal seizures - twitching of left side of face and eye, with increased salivation, and no impairment of consciousness. She was hyperglycaemic 19 mmol/L (normal range: 3.9 - 6.1 mmol/L), and she had no ketones in her urine. The serum electrolytes were all within normal ranges, and her calculated serum osmolality was elevated at 304 mmol/kg.
On day 6, she was noted to have a left facial palsy in keeping with an upper motor neuron lesion, as well as rigidity of her left upper limb.
An urgent computer tomography (CT) brain scan showed right basal ganglia (BG) hyperdensities (Fig. 1), with no cerebral oedema.
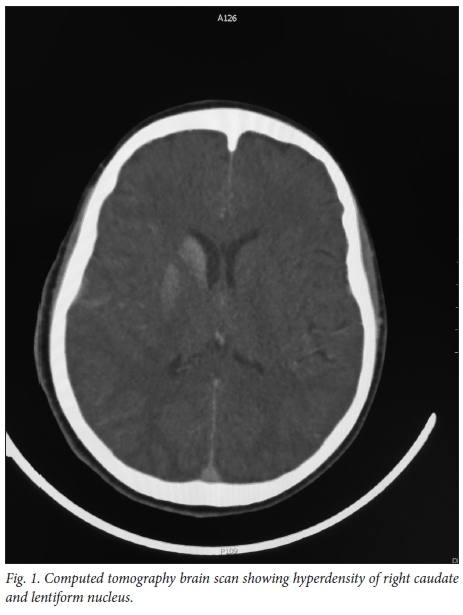
The possible aetiologies entertained in view of her comorbid type 1 diabetes were infective (early abscess and cerebritis), a connective tissue disorder with associated vasculitis or a haemorrhage.
Over the next 48 hours, the facial weakness improved; however, the rigidity and dystonia persisted in her left arm, and she developed left-sided choreoathetoid movements, which progressed to hemiballismus.
To better delineate the BG involvement, magnetic resonance imaging (MRI) of the brain was obtained. The images (Fig. 2 A - C) showed diffuse high T1 signal intensity of the right basal ganglia, with involvement of the caudate nucleus and the putamen. The diffusion weighted (DW) imaging and apparent diffusion coefficient (ADC) mapping showed no evidence of hypoxic/ ischaemic injury. There was no associated mass effect, oedema or volume loss, as would have been expected in a haemorrhage, and the internal capsule was spared.
In view of the above, a diagnosis of non-ketotic hyperglycaemic hemichorea was made - serum glucose of 19 mmol/L, no ketones and with classic clinical and radiological findings.
The patient was started on risperidone to control the debilitating hemiballismus, and there was a marked reduction in the frequency and severity of the movements, with control of her hyperglycaemia. Two weeks after discharge, only occasional movements of her left hand were noted.
A repeat MRI, 4 months after the initial presentation, was normal, with complete resolution of the hyperintensities seen. Clinically, the child was doing well at the diabetes clinic, with good glycaemic control. She was no longer taking the risperidone, and having no abnormal movements. Her neurological exam was unremarkable, with no facial asymmetry and normal tone in all limbs.
Discussion
Hemichorea-hemiballismus is a rare involuntary movement disorder involving both the proximal and distal groups of muscles. The most common associations are lesions of the contralateral subthalamic nuclei or its connections, due to vascular lesions, tumours, HIV infection, toxins or a metabolic disturbance. Sydenham's chorea is an important consideration as well. Chorea-ballismus is an unusual presentation of diabetes, and can either be a result of non-ketotic hyperglycaemia (NKH), the initial presentation of diabetes or due to hypoglycaemia.[3]
NKH is the elevation of blood glucose in the absence of ketone formation. There
are numerous neurological conditions associated with this - delirium/coma, seizures (focal/generalised), hemiparesis, aphasia and nystagmus.[4,5] The association between NKH and hemichorea was first described in 1960.[6]
The diagnosis of NKHHC is that of a classic triad of clinical presentation (hemichorea-hemiballismus), radiological features and clinical resolution of symptoms with glycaemic control.[7]
The clinical characteristics of the movements in NKHHC are that they are unilateral and involuntary, follow a poorly defined pattern and develop over a period of hours. It has been shown to affect older patients, with a predilection for those of Asian descent.[1,8,9] A meta-analysis of 53 cases found the mean age to be 71 (range 22 - 92); there was a female predominance and the majority of cases had both upper and lower limb involvement. The mean serum glucose was 26 mmol/L (range 9 -70 mmol/L).[1] Another review of 9 cases found the condition to occur only in older patients, with a female predilection. This was hypothesised to be a result of decreased levels of oestrogen in post-menopausal women affecting the dopamine function of the nigrostriatal system.[8]
The characteristic radiographic findings are signal changes in the BG contralateral to the side of the patient's symptoms. The differential of the radiographic findings is vast, and includes long-term total parenteral nutrition, Wilson's disease, neurofibro-matosis type 1, hypoxic brain injury, stroke, infection and tumours.[10,11] The CT brain shows unilateral hyperdensity of the putamen and/or caudate nucleus. The T1-weighted MRI images showed high signal intensity BG lesions, with sparing of the internal capsule.[11] The putamen is almost always involved, with variable additional BG lesions. The T2-weighted MRI findings are more variable, with the BG lesions described as either hypointense or isointense to background normal BG. The extent of restriction seen on DW imaging and measured ADC correlate with the severity of damage and the potential for reversibility.
The neuroimaging changes classically resolve completely over time, but lag behind the clinical resolution.
The pathophysiology of NKHHC is poorly understood, and there are two current theories.
The first is that the hyperglycaemia disrupts the blood-brain barrier, causing a decrease in cerebral blood flow, and the second is that hyperviscosity causes hypoperfusion of the striatum. As an alternate energy substrate to ketones, Y-aminobutyric acid (GABA) is metabolised, and the resultant decreased activity has been proposed as the cause of the abnormal movements.[1] The predilection for the basal ganglia can be explained by the abundance of astrocytes and large metabolic demand of this area, with the classic MRI findings thought to occur as a result ofthe gemistocyte (swollen, reactive astrocyte) accumulation, hyperviscosity, neuronal dysfunction and possible cytotoxic oedema.[10,12,13]
The associated movement disorders are not surprising. The components of the basal ganglia together form a functional circuit located as a part of the deep grey matter of the cerebral hemispheres. The circuitry controls movement through the pallidosubthalamic pathways' influence on the thalamocortical tracts. Dysfunction within this circuit will result in a variety of different movement disorders. The combination of hemichorea-hemiballismus comprises of more proximal, higher-amplitude movements (ballismus and lower-amplitude, more distal movements (chorea).[10]
The treatment of NKHHC is aggressive glycaemic control. Once controlled, the hemichorea-hemiballismus settles, in the majority of cases.[14] In refractory cases, or those patients with debilitating ballismus, drugs that block the postsynaptic dopamine receptors, such as haloperidol or risperidone, can be used. There have been reports of improvement with the use of topiramate, most likely through its GABAergic properties.[4]
The long-term prognosis is excellent.[1,9,14] One large series reported that 74% of patients had complete resolution of chorea after a period ranging from 1 day to 10 months; the majority reached full recovery by 6 months and, of these, 30% of cases settled purely with oral glycaemic control.[1] A second case series of 10 patients reported resolution of dyskinesia within days after control of hyperglycemia, in all but 1 patient.[15] The risk of recurrence is low, and in all reported cases it is associated with hyperglycaemia.[1]
Conclusion
NKHHC is a rare condition in the paediatric population. However, the classic clinical and radiological findings, with the excellent long-term prognosis following prompt glycaemic control, makes awareness of this condition important. We suggest that hyperglycaemia should be considered as a differential diagnosis in patients presenting with hemichorea-hemiballismus, and patients presenting with this movement disorder should have their serum glucose levels measured.
Acknowledgements. None.
Author contributions. MPKH: conceptualisation, review, write-up and editing; JLR: write-up and editing.
Funding. None.
Conflicts of interest. None.
References
1. Oh SH, Lee KY, Im JH, Lee MS. Chorea associated with non-ketotic hyperglycemia and hyperintensity basal ganglia lesion on T1-weighted brain MRI study: A meta-analysis of 53 cases including four present cases. J Neurol Sci 2002;200(1-2):57-62. https://doi.org/10.1016/s0022-510x(02)00133-8 [ Links ]
2. Aquino JH, Spitz M, Pereira JS. Hemichorea-hemiballismus as the first sign of type 1b diabetes during adolescence and its recurrence in the setting of infection. J Child Neurol 2015;30(10):1362-1365. https://doi.org/10.1177/0883073814553972 [ Links ]
3. Piccolo I, Sterzi R, Thiella G. Chorea in hyperglycemia. Diabetes Care 1998;21(10):1777. https://doi.org/10.2337/diacare.21.10.1777a [ Links ]
4. McCullen MK, Miller J, Jabbour S, et al. Chorea in the setting of hyperglycaemia -a case report and review of the literature. Pract Neurol 2010:16-19. [ Links ]
5. Maccario M. Neurological dysfunction associated with nonketotic hyperglycemia. Arch Neurol 1968;19(5):525-534. https://doi.org/10.1001/archneur.1968.00480050095009 [ Links ]
6. Bedwell SF. Some observations on hemiballismus. Neurology 1960;10:619-622. https://doi.org/10.1212/wnl.10.6.619 [ Links ]
7. Qi X, Yan YY, Gao Y, Zheng ZS, Chang Y. Hemichorea associated with non-ketotic hyperglycaemia: A case report. Diabetes Res Clin Pract 2012;95(1):e1-3. https://doi.org/10.1016/j.diabres.2011.09.020 [ Links ]
8. Lin JJ, Chang MK. Hemiballism-hemichorea and non-ketotic hyperglycaemia. J Neurol Neurosurg Psychiatry 1994;57(6):748-750. https://doi.org/10.1136/ jnnp.57.6.748 [ Links ]
9. Lin JJ, Lin GY, Shih C, Shen WC. Presentation of striatal hyperintensity on T1-weighted MRI in patients with hemiballism-hemichorea caused by non-ketotic hyperglycemia: Report of seven new cases and a review of literature. J Neurol 2001;248(9):750-755. https://doi.org/10.1007/s004150170089 [ Links ]
10. Hansford BG, Albert D, Yang E. Classic neuroimaging findings of non-ketotic hyperglycaemia on computed tomography and magnetic resonance imaging with absence of typical movement disorder symptoms (hemichorea-hemiballism). J Radiol Case Rep 2013;7(8):1-9. https://doi.org/10.3941/jrcr.v7i8.1470 [ Links ]
11. Wilson TJ, Than KD, Stetler WR Jr., Heth JA. Non-ketotic hyperglycemic chorea-hemiballismus mimicking basal ganglia haemorrhage. J Clin Neurosci 2011;18(11):1560-1561. https://doi.org/10.1016/j.jocn.2011.03.010 [ Links ]
12. Chu K, Kang DW, Kim DE, Park SH, Roh JK. Diffusion-weighted and gradient echo magnetic resonance findings of hemichorea-hemiballismus associated with diabetic hyperglycemia: A hyperviscosity syndrome? Arch Neurol 2002;59(3):448-452. https://doi.org/10.1001/archneur.59.3.448 [ Links ]
13. Cheema H, Federman D, Kam A. Hemichorea-hemiballismus in non-ketotic hyperglycaemia. J Clin Neurosci 2011;18(2):293-294. https://doi.org/10.1016/j.jocn.2010.04.036 [ Links ]
14. Shalini B, Salmah W, Tharakan J. Diabetic non-ketotic hyperglycemia and the hemichorea-hemiballism syndrome: A report of four cases. Neurol Asia 2010;15(1):89-91. [ Links ]
15. Lai PH, Tien RD, Chang MH, et al. Chorea-ballismus with non-ketotic hyperglycaemia in primary diabetes mellitus. Am J Neuroradiol 1996;17(6):1057-1064. [ Links ]
 Correspondence:
Correspondence:
M P K Hauptfleisch
marc.hauptfleisch@wits.ac.za
Accepted 17 May 2018














