Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.12 no.2 Pretoria Abr./Jun. 2018
http://dx.doi.org/10.7196/sajch.2018.v12i2.1470
RESEARCH
The role of kidney injury molecule-1, interleukin-18 and glutathione-S-transferase-π in paediatric HIV-associated nephropathy
L NandlalI; R BhimmaII; T NaickerI
IMMedSci, PhD; Department of Optics and Imaging, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIPhD, MD; Department of Paediatrics and Child Health, College of Health Sciences, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND. HIV-associated nephropathy (HIVAN) in sub-Saharan Africa remains a significant cause of morbidity and mortality in children. Early detection of kidney injury is essential for injury-specific interventions that may avert permanent kidney damage and delay progression of kidney injury. Kidney biopsy is presently the gold standard for diagnosis of related kidney disease; however, it is pervasive with attendant complications, and may not be representative owing to sampling error. Serum creatinine is an insensitive and non-specific marker for the diagnosis of various kidney diseases, particularly in HIV-infected patients, who usually have varying degrees of muscle wasting. Therefore, a non-invasive approach using additional biomarkers for early detection of HIV-related kidney diseases, particularly HIV-associated nephropathy (HIVAN), is urgently needed.
OBJECTIVE. To determine the urinary concentrations of kidney injury molecule-1 (KIM-1), interleukin-18 (IL-18) and glutathione-S-transferase-π (GST-π) in children with idiopathic focal segmental glomerulosclerosis (FSGS) and HIVAN.
Methods. The study group comprised 34 children: 13 with HIVAN and 21 with idiopathic FSGS. The control groups were 19 HIV-positive and 16 HIV-negative children with no kidney disease. Urine samples collected from these 69 children were stored at -80°C. Urinary concentrations of KIM-1, IL-18 and GST-π were quantified using Bio-Plex assay.
RESULTS. A significant increase in urinary KIM-1 levels was observed in the HIVAN group compared with the HIV-positive (p=0.0039) and HIV-negative (p=0.0438) control groups. There was no significant increase in KIM-1 levels on comparison of the idiopathic FSGS group with the control groups (HIV-positive and HIV-negative children) (p=0.0737 and p=0.1757, respectively). No statistically significant differences were noted in urinary IL-18 and GST-π levels across all study groups.
CONCLUSION. Urinary KIM-1 levels are significantly elevated in children with HIVAN and may be a useful biomarker to detect kidney disease in HIV-1-infected children.
The ravages of the HIV-1 epidemic in sub-Saharan Africa persist, despite stringent HIV screening and combined antiretroviral therapy (cART) rollout programmes. Globally, 150 000 children were newly infected with HIV in 2015, albeit a decrease from the 490 000 children in 2000.1 In 2013, approximately 3.2 million children under the age of 15 years were living with HIV infection, of whom 90% were from sub-Saharan Africa.1 At the time, an alarming 70% of these children were not on appropriate cART management.1,2 It is well established that HIV-infected children are at greater risk of developing kidney disease than children without HIV-infection.3 HIV-associated nephropathy (HIVAN), one of the most common manifestations of HIV-related kidney diseases, remains a significant cause of morbidity and mortality in children, particularly in Africa1. Currently, the only definitive way to diagnose HIVAN is by kidney biopsy, an invasive procedure that requires only a small portion of the kidney. However, there are several factors that limit the utility of a kidney biopsy. Sampling may be inadequate, the site of pathology may not be represented and the procedure, being invasive, is not without attendant complications.4 To date, non-invasive strategies for detecting and monitoring the effect of kidney diseases in children primarily depend on: (i) abnormal urine sediments, including the presence of renal tubular epithelial and cast cells;5(ii) random urinary protein to creatinine ratio >1.0 mg/mg;6 (iii) tubular disorders resulting in abnormalities in fluid and electrolyte balance; (iv) reduced glomerular filtration rate (GFR) <60 mL/min/1.73 m2; and (v) an elevated serum creatinine level based on cut-offs that vary with age. Several factors are posed against the utility of serum creatinine levels as these values are influenced by body weight, nutritional status, protein intake and muscle mass, all of which are affected in HIV-infected children.7,8 Additionally, proteinuria can emanate from a variety of non-pathological factors, i.e. emotional stress, physical exertion, fever or orthostatic (postural) proteinuria.9Therefore the identification of novel biomarkers to aid definitive and early diagnosis of HIVAN could significantly influence the clinical care of HIV-infected children, as early institution of cART has been shown to improve the clinical outcome and survival.
Soler-Garcia et al. 10 assessed the value of 11 urinary proteins in children with HIVAN compared with HIV-infected children with no kidney disease. Although these proteins were elevated in children with HIVAN, no significant biomarker was reported from this study in predicting HIVAN. Previous studies reported neutrophil gelatinase-associated lipocalin (NGAL) as an established biomarker of HIVAN in adults11 and a urinary biomarker profile comprised increased levels of fibroblast growth factor-2 (FGF-2) and matrix metalloproteinase-2 (MMP-2), and decreased levels of epidermal growth factor (EGF) was shown to be useful in identifying HIVAN in children.10,12 Despite these contributions, new candidate biomarkers for children with HIVAN are urgently needed to improve the predictive value of biomarkers in the detection of HIVAN.
Kidney injury molecule-1 (KIM-1) is a type I transmembrane glycoprotein, located on the apical membrane of dilated proximal tubules with a cleavable ectodomain (90 kDa).13,14 It is negligibly expressed in normal kidneys (<1 mg/mL) yet is rapidly elevated and expressed in response to various types of kidney disease (3 - 7 ng/mL).13
Interleukin-18 (IL-18), a member of the IL-1 family of cytokines, is synthesised as an inactive 23 kDa precursor by several tissues including proximal tubular epithelial cells, macrophages and monocytes.15 Moreover, urinary IL-18 levels are elevated in patients with acute kidney injury and delayed graft function compared with normal subjects.16 Recent studies have also focused on the diagnostic accuracy of IL-18 levels in predicting idiopathic focal segmental glomerulosclerosis (FSGS).15
Glutathione-S-transferase (GST-π) is a soluble cytosolic enzyme that indicates distal tubular injury.17 Increased levels of GST-π in the urine after nephrotoxic injury are attributed to leakage from the tubular epithelial cells into the tubular lumen secondary to cell damage.18 Leakage and increased expression of this urinary protein serves as an important biomarker of FSGS.
In an attempt to evaluate the accuracy of KIM-1, IL-18 and GST-π as predictors of kidney disease in HIV-infected children, notably HIVAN, we compared and contrasted the urinary levels of KIM-1, IL-18 and GST-π in children with HIVAN and idiopathic FSGS with children (HIV positive and HIV negative) with no kidney disease.
Method
Study design
Ethical approval (ref. no. BE094/16) to conduct the study was obtained from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal and informed consent was obtained from the parent or guardian and assent (where applicable) from the patient. Urine samples collected for another study (ref. no. BE321/13) from children attending the Inkosi Albert Luthuli Central Hospital in Durban, KwaZulu-Natal, South Africa, were used for the study. Samples were collected about 2 - 4 years after the kidney biopsy was done and stored at -80°C for a period of about 2 months until analysed using Bio-Plex.
Study population
All children included in the study were black African children between 1 and 16 years. The study group (N=34) consisted of children with biopsy-proven HIVAN (n=13) and idiopathic FSGS (n=21). Comorbidity in the children with HIVAN included chronic lung disease (n=3), cardiomyopathy (n=5) and stunting (n=5). None had fever or other evidence of secondary infections at the time of sample collection. The control group (N=35) consisted of children who were HIV positive with no kidney disease (n=19) and HIV negative with no kidney disease (n=16). The latter group comprised HIV-negative children recruited from follow-up clinics with no kidney disease, e.g. respiratory, neurology and endocrine clinics.
All 13 children with HIVAN were on cART and angiotensin-converting enzyme antagonists for a minimum of 2 years before recruitment. The 21 children with idiopathic FSGS (HIV negative) were on low-dose steroid, angiotensin inhibitors as well as additional immunonosuppressants such as calcinuerin inhibitors (cyclosporin or tacrolimus) or pulse doses of methylprednisolone at the time of sample collection. Children who were below the age of 1 year or over 16 years, HIV-positive children with kidney disease but absence of kidney biopsy or inadequate histology, and those with histological forms of nephrotic syndrome (NS) other than FSGS were excluded. Urine was aliquoted and stored in cryovials at -800C until analysis.
Diagnosis of HIVAN
The diagnosis of HIVAN was made following confirmation of HIV-1 infection and presence of persistent proteinura ≥1+ on urinary dipstick examination (on at least 3 separate occassions in non-febrile children) with one or more of the following: (i) presence of enlarged echogenic kidneys by renal ultrasound; (ii) abnormal urinary sediment; (iii) microcystic tubular dilation, a childhood variant of HIVAN in the absence of significant podocyte lesions; and (iv) histological finding of FSGS.19,20
Multiplex method
The urine samples were analysed for KIM-1, IL-18 and GST-π using the quantitative Bio-Plex Pro RBM Kidney Toxicity Assay (Panel 1) (Bio-Rad Laboratories, USA) according to the manufacturer's instructions21 The analysis of each sample was performed by means of low photomultiplier tubes (PMT) (Bio-Plex 200). Data were collected and analysed using a BioPlex 200 instrument equipped with Bio-Plex Manager analysis software version 4.1. A standard curve was generated using the known concentration (ng/mL) of each analyte by plotting the median fluorescent intensity (MFI) signal against concentration. These standards were used to interpolate the concentration of the unknown samples. Intra-plate variability was determined with CV <20% and

between 70% and 130% (r=0.8, p=0.05). The data were imported into an Excel spreadsheet for statistical analysis.
Statistical analysis
All statistical analysis was undertaken using GraphPad Prism version 5 (GraphPad, USA). To analyse non-normal data, we used the non-parametric t -test (Mann-Whitney U). One-way ANOVA was used to correct for the multiple comparisons among the four study groups. Spearman coefficients were used to evaluate correlations between biomarkers. A p-value <0.05 was considered as statistically significant. Graphical data were represented as median and interquartile range.
Results
All 34 children with biopsy proven FSGS had a histopathological pattern of FSGS not otherwise specified according to the Columbian classification.22 Thirteen children (19%) were HIV-positive and were confirmed paediatric HIVAN whilst 21 (30%) children had idiopathic FSGS. The mean (SD) age for HIVAN and idiopathic FSGS was 14 (2.73) (range 8.2 - 16.3) and 10 (3.40) (range 4.1 - 16.4) years respectively. All patients presented with NS. The control group consisted of 35 children with no kidney disease; 16 children (23%) were HIV negative with a mean (SD) age of 5 (3.43) (range 1 - 11) years and 19 children (28%) were HIV-positive with a mean (SD) age of 11 (3.52) (range 5 - 15) years (Table 1).
The patients with established FSGS had stages 1 and 4 chronic kidney disease (CKD) according to the KDIGO classification.8 In the idiopathic FSGS group, 14 patients had CKD stage 1; 4 stage 2; 2 stage 3; and 1 stage 4. In the HIVAN group, 10 patients were CKD stage 1, 1 stage 2, and 2 stage 4. Based on the WHO Disease Staging System for HIV Infection and Disease in Children,23 10 children were totally asymptomatic (Clinical Stage I) and 3 patients had persistent proteinuria (Clinical Stage 4). The latter patients were diagnosed with FSGS for a mean of 2.8 years with a range of 2.1 - 4.3 years prior to study entry. Kidney biopsy showed FSGS (not otherwise specified) in all patients with over 80% of glomeruli having more than 50% sclerosis.
To identify associations with the variability, we compared urinary protein concentration of KIM-1, IL-18 and GST- π with age, weight, creatinine, urea, albumin, cholesterol and estimated glomerular filtration rate (eGFR) in the four groups of children. No significant correlations were observed between KIM-1, IL-18 and GST-π
Urinary concentration of KIM-1, IL-18 and GST-π
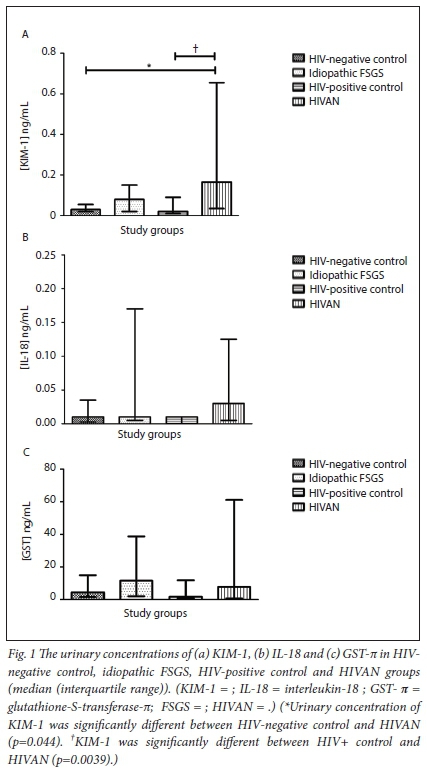
The urinary concentration of KIM-1, IL-18 and GST-π are displayed in Figs 1A, 1B and 1C, respectively. There was a significant increase of KIM-1 in the HIVAN group (mean 0.52 ng/mL; 95% confidence interval (CI) 0.035 - 0.0033) compared with the HIV positive control group (mean 0.048 ng/mL; 95% CI 0.074 - 0.022) (Mann-Whitney U=74.00; p=0.0039). KIM-1 levels were up-regulated in the idiopathic FSGS group (mean 0.17 ng/mL; 95% CI 0.29 - 0.050) compared with the HIV-negative control group (mean 0.072 ng/mL; 95% CI 0.14 - 0.0060); however, this did not reach statistical significance (Mann-Whitney U=88.00; p=0.18). There was also an increase in KIM-1 levels in the idiopathic FSGS group (mean 0.17 ng/mL; 95% CI 0.29 - 0.050) compared with the HIV-positive control group (mean 0.048 ng/mL; 95% CI 0.074 - 0.022) but once again this did not reach statistical significance (Mann-Whitney U=116.0; p=0.074). Although KIM-1 levels were higher in children with HIVAN (mean 0.52 ng/ mL; 95% CI 0.035 - 0.0033) compared with children with idiopathic FSGS (mean 0.17 ng/mL; 95% CI 0.29 - 0.050), this increase was also not significant (Mann-Whitney U=114.5; p=0.22). There was a significant increase of KIM-1 in the HIVAN group (mean 0.52 ng/ mL; 95% CI 0.035 - 0.0033) compared with the HIV-negative control group (mean 0.072 ng/mL; 95% CI 0.14 - 0.0060) (Mann-Whitney U=58.00; p=0.044). No statistically significant differences of IL-18 and GST-π levels were noted across the study groups (Table 2).
Discussion
In a large paediatric study conducted across a spectrum of HIV-related kidney diseases in children from the province of KwaZulu-Natal, we showed a 65.3% predominance of FSGS, of which cases 26.5% had collapsing glomerulopathy while 38.8% were the classical variant of FSGS (not otherwise specified).20,22 In the present study, we report 3 candidate biomarkers (KIM-1, IL-18 and GST-π) in HIVAN (all with the classical variant of FSGS on histopathology) in children compared with idiopathic FSGS and HIV-positive and HIV-negative controls.
The only statistically significant increase was noted in urinary KIM-1 levels in the HIVAN group compared with the HIV-positive and HIV-negative control groups. In contrast, there was no significant difference in KIM-1 levels between the idiopathic FSGS group compared with the HIV-positive and HIV-negative control groups. These results indicate that KIM-1 is up-regulated in children with HIVAN. Our results are corroborated by previous studies that report a dramatic increase of KIM-1 in patients who develop CKD in contrast to the low expression of this biomarker in healthy individuals.14,24 Notably, using histopathology as the gold standard, KIM-1 was found to have the highest sensitivity and specificity amongst 21 urinary biomarkers studied in identifying kidney injury.25-27
To date, strategies for monitoring the effect of HIV on the kidney primarily depend on the surveillance of serum creatinine concentrations, which is an indicator of GFR rather than injury. Previous studies have shown that KIM-1 could be used as a urinary biomarker of kidney injury. Therefore it has been used to monitor the impact of HIV infection on the kidney, as well as the effect of antiretroviral therapy (tenofovir) that causes proximal tubular damage.28,29
The severity of pathology in CKD has also been associated with elevated urinary KIM-1 levels.30,31 However, in our study, KIM-1 levels were not significantly elevated in children with idiopathic FSGS compared with HIV-positive and -negative children with no kidney disease (control). As KIM-1 is site specific, it is markedly expressed by proximal tubular cells in response to injury by shedding its ectodomain into the tubular lumen, and therefore may be particularly beneficial for detecting HIV-related kidney injury.28 As KIM-1 may be an early marker of kidney injury emanating from damage to proximal tubular cells, in patients with CKD with established fibrosis the degree of ongoing tubular injury may have progressed to the stage where it may not significantly elevate levels of KIM-1. This possibly explains the slightly increased levels, albeit not statistically significant, in children with idiopathic FSGS with established disease compared with controls. Also, it is possible that the small sample size may be the reason that we did not detect significant differences across both study groups and controls.
KIM-1 is probably not specific for HIV infection as indicated in our study, as it was not significantly different in children with HIVAN compared with the idiopathic FSGS group. In the light of these findings, kidney biopsy will be required to confirm FSGS or other histological forms of kidney disease associated with HIV. In addition, our study did not determine the differences in KIM-1 between HIVAN and other histological forms of HIV-related kidney disease. We submit that although our study showed a significant difference in KIM-1 levels between children with HIVAN, and both HIV-positive and HIV-negative controls, its sensitivity and specificity will need to be assessed in larger studies and its use will need to be compared with other non-specific biomarkers such as microalbumin which is much cheaper and readily available.
A study conducted by Kilis-Pstrusinska et al.30 has reported elevated levels of urinary IL-18 in idiopathic nephrotic syndrome (NS). The results of the above study indicated the relationship between the active phase of idiopathic NS and levels of IL-18, thus suggesting the role of IL-18 in the pathogenesis of idiopathic NS as well as its association with the activity of the disease. Similarly, Deebii et al.31 also reported that IL-18 can be used as an early marker of subclinical kidney tubular dysfunction in patients who are HIV-infected. IL-18 increases in urine only under conditions of marked tubular damage, apoptotic tubular cell shedding, and cell necrosis, all of which are associated with deterioration of kidney function. Our study reports an increase in IL-18 in children with HIVAN and idiopathic FSGS compared with the controls (HIV-positive and HIV-negative), albeit non-significant. This finding may also be attributed to the small sample size used in our study, making it difficult to detect significant differences across the groups. Also, as IL-18 increases in the urine only under conditions of marked tubular damage, cell necrosis and apoptotic tubular cell shedding, this may have not been the case in our children who already had established disease.
Urinary GST-π excretions were also reported to be useful biomarkers of renal tubular injury.32 GST-π is site specific, generally not present in the urine of normal subjects, but is markedly up-regulated in the distal tubular cells during renal injury.33 Once again, we report a non-significant upwards trend in urinary GST-π levels in our study, although the levels were elevated in children with idiopathic FSGS compared with the controls. This lack of significant differences in GST-π levels between the study group and controls may again also be attributed to sample size or that our patients had established disease and were on treatment with arrested or markedly attenuated distal tubular cell injury.
There were few other limitations in our study, which was a retrospective study with urine samples stored at -80°C to prevent protein degradation. It is possible that with the length of time, storage may have resulted in a decrease of urinary proteins, thus negating any significant differences in urinary levels of the biomarkers we studied in the various groups. This study was a single-centre study in a homogeneous group of black African children and may therefore not be applicable to other population groups. Also, patients recruited into the study were on treatment, which could have affected the levels of urinary biomarkers studied.
Conclusion
The present study has demonstrated that KIM-1 is significantly elevated in children with HIVAN compared with HIV-positive and HIV-negative controls. Larger prospective studies to determine the role of KIM-1 in early detection of HIVAN, thus obviating the need for kidney biopsy, and allowing early institution of appropriate therapy, thereby improving clinical outcome and survival, are needed.
Acknowledgements: The authors thank Albert Luthuli and King Edward VIII Hospitals and all the patients who consented to participate in the study, and the Optics and Imaging Centre, DDMRI, College of Health Sciences, where the study was conducted.
Author contributions. We thank Dr Elaene Naicker for providing some of the study samples and clinical care of the children. R. Bhimma and T. Naicker were supported by the Medical Research Council and National Research Foundation.
Funding. Funding was received from the College of Health Sciences, University of KwaZulu-Natal.
Conflicts of interest. None.
References
1. UNAIDS. http://www.unaids.org/en/resources/fact-sheet. Geneva: UNAIDS, 2015. [ Links ]
2. UNAIDS. Gap Report. http://www.unaids.org/en/resources/documents/2016/prevention-gap. Geneva: UNAIDS, 2016. [ Links ]
3. Pezzaro S, Soler-Garcia AA, Hathout Y, Jharma R.D, Ray PE. Urinary biomarkers of kidney diseases in HIV-infected children. Proteomics Clin Appl 2015; 9(5-6):490-500. https://doi.org/10.1002/prca.201400193. [ Links ]
4. Cameron JS, Hicks J. The introduction of renal biopsy into nephrology from 1901 to 1961: A paradigm of the forming of nephrology by technology. Am J Nephrol 1997;17(3-4):347-358. https://doi.org/10.1159/000169122 [ Links ]
5. Ray PE, Rakusan T, Loechelt BJ, Selby DM, Liu XH, Chandra S. Human immunodeficiency virus (HIV)-associated nephropathy in children from the Washington, D.C. area: 12 years' experience. Semin Nephrol 1998;18(4):396-405. [ Links ]
6. Chaparro AI, Mitchell CD, Abitbol CL, et al. Proteinuria in children infected with the human immunodeficiency virus. J Pediatr 2008;152(6):844-849. https://doi.org/10.1016/j.jpeds.2007.11.007 [ Links ]
7. Coca SG, Parikh CR. Urinary biomarkers for acute kidney injury: Perspectives on translation. Clin J Am Soc Nephrol 2008;3(2):481-490. https://doi.org/10.2215/CJN.03520807 [ Links ]
8. Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 2014;85(1):49-61. https://doi.org/10.1038/ki.2013.444 [ Links ]
9. Leung AK, Wong AH. Proteinuria in children. Am Fam Physician 2010;82(6):645-651. [ Links ]
10. Soler-Garcia AA, Rakhmanina NY, Mattison PC, Ray PE. A urinary biomarker profile for children with HIV-associated renal diseases. Kidney Int 2009;76(2):207-214. https://doi.org/10.1038/ki.2009.115 [ Links ]
11. Paragas N, Nickolas TL, Wyatt C, et al. Urinary NGAL marks cystic disease in HIV-associated nephropathy. J Am Soc Nephrol 2009;20(8):1687-1692. https://doi.org/10.1681/ASN.2009010065 [ Links ]
12. Kiley SC, Chevalier RL. Urinary biomarkers: the future looks promising. Kidney Int 2009;76(2):133-134. https://doi.org/10.1038/ki.2009.124 [ Links ]
13. Waanders F, van Timmeren MM, Stegeman CA, Bakker SJL, van Goor H. Kidney injury molecule-1 in renal disease. J Pathol 2010;220(1):7-16. https://doi.org/10.1002/path.2642 [ Links ]
14. Boettner, B. KIM-1 driving chronic kidney disease. SciBX 2013;39(6):1-2. https://doi.org/10.1038/scibx.2013.1085 [ Links ]
15. Nickolas TL, Barasch J, Devarajan P. Biomarkers in acute and chronic kidney disease. Curr Opin Nephrol Hypertens 2008;17(2):127-132. https://doi.org/10.1097/MNH.0b013e3282f4e525 [ Links ]
16. Hall IE, Yarlagadda SG, Coca SG, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol 2010;21(1):189-197. https://doi.org/10.1681/ASN.2009030264 [ Links ]
17. Branten AJ, Mulder TP, Peters WH, Assmann KJ, Wetzels JF. Urinary excretion of glutathione S transferases alpha and pi in patients with proteinuria: Reflection of the site of tubular injury. Nephron 2000;85(2):120-126. https://doi.org/10.1159/000045644 [ Links ]
18. Harrison DJ, Kharbanda R, Cunningham DS, McLellan LI, Hayes JD. Distribution of glutathione S-transferase isoenzymes in human kidney: Basis for possible markers of renal injury. J Clin Pathol 1989;42(6):624-628. https://doi.org/10.1136/jcp.42.6.624 [ Links ]
19. Senguttuvan P, Gowtham S, Soundararajan P. Human immunodeficiency virus-associated nephropathy (HIVAN) in Indian children. Open Urol Nephrol J 2014;(1):1. https://doi.org/10.2174/1874303X01407010105 [ Links ]
20. Ramsuran D, Bhimma R, Ramdial PK, et al. The spectrum of HIV-related nephropathy in children. Pediatr Nephrol 2012;27(5):821-827. https://doi.org/10.1007/s00467-011-2074-8 [ Links ]
21. BioRad Laboratories. Bio-Plex Pro RBM Human Kidney Toxicity Assays. California: BioRad Laboratories, 2016. [ Links ]
22. DAgati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 2004;43(2):368-382. https://doi.org/10.1053/j.aj9kd.2003.10.024 [ Links ]
23. World Health Organization. www.who.int/hiv/pub/guidelines/HIVstaging150307. Geneva: World Health Organization, 2007. [ Links ]
24. Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: A sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 2006;290(2):517-529. https://doi.org/10.1152/ajprenal.00291.2005 [ Links ]
25. Dieterle F, Staedtler F, Grenet O, Cordier A, Perentes E. Qualification of biomarkers for regulatory decision making - a kidney safety biomarker project. Toxicol Off J Soc Toxicol 2007;96:381. [ Links ]
26. Han WK., Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney injury molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int 2002;62(1):237-244. https://doi.org/10.1046/j.1523-1755.2002.00433.x [ Links ]
27. Bonventre JV. Kidney injury molecule-1 (KIM-1): A urinary biomarker and much more. Nephrol Dialysis Transplant 2009;24(11):3265-3268. https://doi.org/10.1093/ndt/gfp010 [ Links ]
28. Shlipak MG, Scherzer R, Abraham A, et al. Urinary markers of kidney injury and kidney function decline in HIV-infected women. J Acquir Immune Defic Syndr 1999;61(5):565. https://doi.org/10.1097/QAI.0b013e3182737706 [ Links ]
29. Scherzer R, Estrella M, Li Y, Deeks SG, Grunfeld C, Shlipak MG. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS 2012;26(7):867. https://doi.org/10.1097/QAD.0b013e328351f68f [ Links ]
30. Kilis-Pstrusinska K, Medynska A, Zwolinska D, Wawro A. Interleukin-18 in urine and serum of children with idiopathic nephrotic syndrome. Kidney Blood Press Res 2008;31(2):122-126. https://doi.org/10.1159/000124284 [ Links ]
31. Deebii NTI, Orluwene CG, Okerengwo AA, Obunge OK, Odum EP, Oko-jaja RI. Early diagnosis of renal tubular dysfunction in HIV infected patients; a case of interleukin (IL)-18 and other common indicators of renal toxicity. Immunother Open Acc 2016;2:118. https://doi.org/10.4172/2471-9552.100011 [ Links ]
32. Bashir M, Cawood T, O'Shea D, Lawless L, Brady J, Murray B. Obesity-related nephropathy; evidence of proximal tubular damage. Endocrine Abstracts 2008;15:123. [ Links ]
33. de Geus HR, Bakker J. Biomarkers for the prediction of acute kidney injury: A narrative review on current status and future challenges. Clin Kidney J 2012;2(5):102-108. https://doi.org/10.1093/ckj/sfs008 [ Links ]
 Correspondence:
Correspondence:
L Nandlal
loun0406@gmail.com
Accepted 18 January 2018














