Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Child Health
versión On-line ISSN 1999-7671
versión impresa ISSN 1994-3032
S. Afr. j. child health vol.12 no.1 Pretoria ene./mar. 2018
http://dx.doi.org/10.7196/sajch.2018.v12i1.1245
RESEARCH
Retrospective review of neonates with persistent pulmonary hypertension of the newborn at Charlotte Maxeke Johannesburg Academic Hospital
I HarerimanaI; D E BallotII; P A CooperIII
IMB ChB, MMed, FCPaed (SA); Department of Paediatrics and Child Health, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIMB ChB, FCPaed (SA), PhD; Department of Paediatrics and Child Health, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIIMB ChB, FCPaed (SA), PhD; Department of Paediatrics and Child Health, School of Clinical Medicine, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND. Persistent pulmonary hypertension of the newborn (PPHN) is a clinical syndrome characterised by high pulmonary pressures, low systemic pressures and severe hypoxaemia due to circulation transition failure after birth.
OBJECTIVE. To determine the incidence of and describe the risk factors, infant characteristics and treatment strategies for PPHN at Charlotte Maxeke Johannesburg Academic Hospital over the last 8 years.
METHODS. This was a retrospective descriptive study. Patient records of neonates who had a discharge diagnosis of PPHN were reviewed for the period from January 2006 to December 2013. Neonates' PPHN diagnosis was based on clinical criteria and, where possible, echocardiography. Neonates with a congenital cyanotic heart defect were excluded.
RESULTS. A total of 81 neonates had a discharge diagnosis of PPHN, of whom 72 were included in the study. Of the 72 neonates, 37 (51.4%) were female, 38 (52.8%) were born by vaginal delivery and 44 (61.1%) were inborn. The mean (standard deviation (SD)) birth weight was 2.94 (0.69) kg while the mean (SD) gestational age was 38.2 (3.3) weeks. Meconium aspiration syndrome (MAS) was seen in 43 neonates (59.7%) and was the most common disease underlying PPHN. Of the 72 neonates, 67 (93.1%) required mechanical ventilation, but only18.1% required high-frequency oscillatory ventilation. Magnesium sulphate and sildenafil were used in 12 (16.7%) and 9 neonates (12.5%), respectively. Inhaled nitric oxide (iNO) and extracorporeal membrane oxygenation treatments were not available. Of the 72 neonates, 25 (34.7%) died. The need for inotropic support was associated with a poor outcome (p=0.01).
CONCLUSION. PPHN was uncommon in our unit, but its management proved challenging owing to the high mortality risk. The leading cause of PPHN was MAS. Consideration should be given to introducing iNO, given that extracorporeal membrane oxygenation (ECMO) treatment is expensive and labour intensive and probably not justified at this time.
Persistent pulmonary hypertension of the newborn (PPHN) is a clinical condition characterised by severe respiratory failure and hypoxaemia.[1] Its incidence is estimated at around 2 per 1 000 live births worldwide and it is associated with a high morbidity and mortality.[2,3] Despite the progress in treating PPHN, it remains a potentially fatal disease, especially in resource-limited settings.[4] The reported overall mortality ranges from 4% to 33% in developed countries[2] and from 25% to 48% in developing countries.[5,6]
In South Africa (SA), previous studies reported the incidence of PPHN to be 1.1%, with a mortality rate of 31% at Tygerberg Children's Hospital[7] and 48% at Chris Hani Baragwanath Academic Hospital.[8] PPHN develops due to the failure of circulatory transition at birth, causing the pulmonary artery pressure to remain higher than systemic pressures.[9] A patent ductus arteriosus (PDA) or the right-to-left shunting of blood through a patent foramen ovale (PFO) results in severe hypoxaemia.[1,10,11] PPHN usually affects term or near-term newborns, although preterm neonatess can also be affected.[1]
PPHN was initially known as persistent fetal circulation, but was later renamed to the current form to better describe its pathophysiology.[11] PPHN is usually secondary to an underlying pulmonary pathology, although primary or idiopathic PPHN also occurs.[1,10,11] Konduri et al.[u]reported that meconium aspiration syndrome (MAS) was the leading cause of PPHN (42%) in a multicentre trial of inhaled nitric oxide (iNO), followed by idiopathic PPHN (27%), respiratory distress syndrome (RDS) (17%), pneumonia or sepsis (13%) and, less frequently, lung hypoplasia. Other potential risk factors for PPHN include perinatal asphyxia, polycythaemia, acidosis and hypothermia.[3]
PPHN is suspected when there is a considerable difference between preductal and postductal oxygen saturation, in combination with severe hypoxaemia that does not improve when the infant is subjected to 100% supplemental oxygen. As it is difficult to differentiate PPHN from cyanotic congenital heart disease on clinical grounds alone, echocardiography is usually required to confirm a diagnosis of PPHN.[1,3]
The survival rate of neonates suffering from PPHN has been improved by the use of high-frequency oscillatory ventilation (HFOV), selective pulmonary vasodilators such as iNO and phosphodiesterase inhibitors (sildenafil and milrinone), surfactant and extracorporeal membrane oxygenation (ECMO).[1,10-15] In resource-limited facilities, sildenafil and magnesium sulphate have been shown to be safe, effective pulmonary vasodilators for improving oxygenation when iNO is not available.[16-19] Adjuvant treatments such as inotropic support, correction of metabolic disturbances and minimal handling also have invaluable roles in the management of these neonates.[11,15] Alkalinisation by hyperventilation has been abandoned because of consequent neurological complications and the increased risk of chronic lung disease.[11,15] The current mainstay of PPHN treatment when conventional ventilatory support alone fails, consists of a combination of HFOV and administering iNO. This combination treatment has been shown to reduce the need for ECMO more effectively than when either of them is used separately.[10,13] However, the treatment does not reduce duration of hospitalisation or the mortality risk[13] and there are still unresolved debates and controversies regarding the appropriate time of initiation and the optimal dose of iNO.[10]
ECMO is used as a rescue therapy for neonates in respiratory failure and who are unresponsive to other therapies.[20,21] Although it is an expensive and labour-intensive treatment, its introduction has markedly improved the outcome of neonates suffering from PPHN in well-resourced centres.[22,23] The use of iNO and ECMO is not currently offered routinely to neonates at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) owing to resource constraints and a lack of equipment, although the cardiothoracic unit does offer ECMO to selected neonates following open-heart surgery.
PPHN can be fatal and MAS, a leading cause of PPHN, is frequent in our setting. As there were no outcome data from our centre, we conducted a retrospective review of the neonatal database at CMJAH to describe the neonates' characteristics, risk factors and treatment modalities associated with PPHN over an 8-year period.
Methods
This was a retrospective, descriptive study of neonates with a discharge diagnosis of PPHN. The sample included both inborn and outborn neonates admitted to the neonatal unit at CMJAH from January 2006 to December 2013.
Neonates with a discharge diagnosis of PPHN were identified from a computerised database at the neonatal unit of CMJAH, where data were initially collected for the purpose of clinical audit using REDCap (Research Electronic Data Capture) software hosted by the University of the Witwatersrand. Retrieved data of both mothers and neonates were supplemented by a review of their medical records if available. Maternal data included age, parity, gravidity, disease during pregnancy, use of nonsteroidal anti-inflammatory drugs and mode of delivery. Infant data included gestation age, birth weight, gender, place of birth, Apgar score, ventilation mode and duration, drug therapy in the intensive care unit (ICU), echocardiographic findings, hospital stay and outcome on discharge. ICU charts were not available and we therefore were unable to determine the severity of respiratory failure based on the oxygenation index.
The diagnosis of PPHN was made on clinical grounds by the attending neonatologist. Factors considered in the diagnosis included differential oxygen saturation >10% (difference between preductal and postductal) or difference in arterial PO2 >20 mmHg, hypoxaemia disproportionate to the chest X-ray changes and unresponsiveness to a hyperoxia test. Owing to limited capacity of paediatric cardiologists at CMJAH, echocardiography to confirm PPHN was performed only in selected cases.
Both preterm and term neonates who fulfilled the criteria were included in this study, whether inborn or outborn. Neonates with congenital diaphragmatic hernia were included in the study whereas those with a cyanotic congenital heart defect on echocardiography were excluded. Neonates for whom adequate data were not available or whose medical records could not be retrieved were excluded.
Neonates had been managed by the attending neonatologist according to unit protocols. All neonates who were clinically diagnosed with PPHN and required ventilation were initially ventilated with conventional mechanical ventilation (CMV); those who did not respond to CMV were changed to HFOV treatment. All neonates on assisted ventilation were sedated with venous boluses of morphine orfentanyl, with or without a benzodiazepine (midazolam). Neonates were generally not paralysed. Only sildenafil and magnesium sulphate were used as pulmonary vasodilators; the unit did not offer iNO or ECMO treatment during the study period.
Adjuvant therapy for PPHN included exogenous surfactant for RDS or severe MAS and haemodynamic support with inotropes (dopamine, dobutamine and adrenaline) when indicated. In the case of severe metabolic acidosis, sodium bicarbonate infusion was used to keep pH >7.25 if ventilation and improved perfusion did not correct the pH balance. Hyperventilation was not used in our unit. General measures included correction of metabolic disturbances when indicated and 'minimal' handling.
Statistics
Data were analysed using STATA version 2, overseen by a statistician. Categorical variables were described according to frequencies and percentages, whereas continuous variables were described using mean and standard deviation or median and range, depending on the data distribution. The Pearson χχ2 test and Fisher's exact test were used for univariate analysis of categorical variables, while Student's t-test or the Mann-Whitney test was used for continuous variables to compare maternal or infant characteristics between neonates who died and those who survived. Statistical significance was accepted at p<0.05.
Ethics
Permission to conduct the study was obtained from the University of the Witwatersrand's Human Research Ethics Committee (ref. no. M130650) and from the Chief Executive Officer of CMJAH.
Results
During the 8-year period, 81 neonates with a discharge diagnosis of PPHN were identified. Of these, 72 were included in the study. Of the remaining 9 neonates, 6 were excluded because they had major congenital heart defects other than a PDA or PFO on echocardiography, 2 were excluded because the relevant data could not be retrieved and 1 was excluded because the discharge diagnosis of PPHN had been allocated erroneously. Demographic characteristics of the neonates are summarised in Table 1.
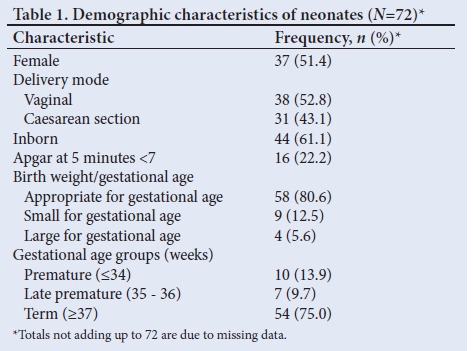
The mean (standard deviation (SD)) birth weight was 2.94 (0.69) kg and the mean (SD) gestational age was 38.2 (3.3) weeks. The mean (SD) maternal age at delivery was 26.2 (5.8) years. The majority of mothers (81.9%) did not have a chronic or pregnancy-related disease predisposing their neonates to PPHN and only 5 (6.9%) had pregnancy-induced hypertension. None of the mothers reported using nonsteroidal anti-inflammatory drugs during pregnancy. A total of 54 neonates (75%) were born at term, with 44 (61.1%) being inborn. Just over half of the neonates were female (51.4%). A similar proportion (52.8%) were born by vaginal delivery. A 5-minute Apgar score <7 was reported for 16 neonates (22%).
Echocardiography to confirm the diagnosis of PPHN by demonstration of right-to-left shunt was performed in only 27 neonates (37.5%). Of these, 10 neonates had either a PDA and PFO or a PDA only with a right-to-left shunt.
MAS was the most common underlying pathology (59.7%), followed by congenital pneumonia and RDS, accounting for 9 (12.5%) and 6 (8.3%) cases, respectively (Table 2).
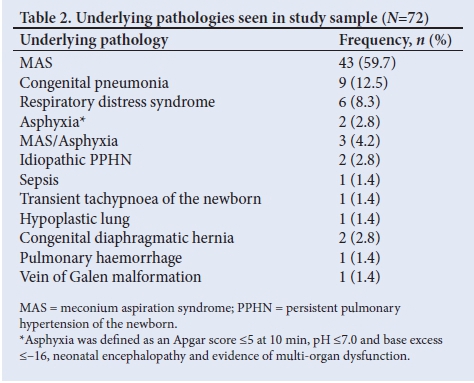
Although CMJAH is a surgical referral centre, only 7 neonates were admitted with congenital diaphragmatic hernia during the study period, 2 of whom had had PPHN; neither survived.
Mechanical ventilation was given to 67 neonates (93.1%) (Table 3). Of these, 13 were unresponsive to CMV and required HFOV The median duration of mechanical ventilation was 4 (range 0 - 31) days. Of the 43 neonates who presented with MAS, only 7 (16.3%) were treated with exogenous surfactant. Magnesium sulphate and sildenafil were used in 12 (16.7%) and 9 (12.5%) neonates, respectively.
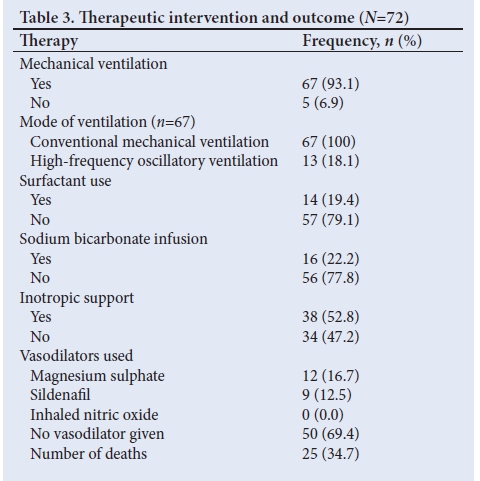
The mortality rate was 34.7% and the majority (62.5%) died within 24 hours of admission. The median duration of hospital stay was 8 (range 0 - 42) days.
The comparison between survivors and nonsurvivors is shown in Tables 4 and 5. The variables analysed were similar between survivors and nonsurvivors, except for the use of inotropes associated with non-survival.
There was no difference between survivors and nonsurvivors with regard to birth weight, 5-minute Apgar scores or gestational age (p>0.05). The duration of both ventilation and hospital stay was longer for the survivors than nonsurvivors (p=0.000), probably because of early death in non-survivors (Table 5).
MAS was the most common associated disease in this study. The characteristics of neonates with MAS are shown in Table 6.

Discussion
MAS accounted for 43 cases (59.7%) of PPHN in this study. Meconium-stained amniotic fluid (MSAF) has been reported to complicate 7 - 22% of term deliveries and up to 52% of postdate deliveries (>41 weeks of gestation).[24] The incidence of MAS has recently declined owing to improved obstetric practices, including reduced postdate deliveries, good intrapartum monitoring of the fetal heart rate and resuscitation of depressed neonates born with MSAF.[25]
Despite remarkable progress in understanding the pathophysiology and treatment of PPHN,[10,11-14] the condition remains a treatment challenge for neonatologists, especially in developing countries, and the associated mortality rate remains high in resource-limited settings.[5-9,26,27-
Of the 72 neonates included in our study, 51.4% were female and 52.8% were born by vaginal delivery. Birth weight was appropriate for gestation age in 80.6% of cases in this study. These results differ from those of previous studies, which reported PPHN to be associated more with males, being large for gestation age and delivery through caesarean section.[5,6,9,26-29] The majority of the neonates included in this study (75%) were born after 37 weeks, the mean gestational age being 38.2 weeks and the mean birth weight 2.94 kg. These results are consistent with evidence that PPHN affects mainly term and post-term neonates.[9,11,29] Only 5 neonates (6.9%) were born to mothers who presented with pregnancy-induced hypertension, a known risk factor for PPHN, although this number may be an underestimation owing to maternal history not always being well documented in the infant's medical records. In our study, the most common underlying diseases in PPHN were MAS, pneumonia and RDS. MAS has repeatedly been reported as the most common lung parenchymal disease resulting in PPHN, followed by idiopathic PPHN and pneumonia or RDS.[1,10,11,13,30] Our results were similar to those reported in previous studies[2,6,7] except that idiopathic PPHN accounted for only 2.8% in our study.
The majority (93.1%) of neonates included in our study were mechanically ventilated (18.1% did not respond to CMV and were switched to HFOV). In a study by Rocha et al.[9]30.7% of neonates were treated with exogenous surfactant whereas only 19.4% of neonates in our study were treated similarly. It is possible that artificial surfactant was used too sparingly in our unit, which might have contributed to the mortality rate.
Our findings are consistent with reports that assisted ventilation constitutes the mainstay of PPHN treatment.[113-15,30] The high proportion of neonates treated with mechanical ventilation in our study may reflect the severity of their disease, although we were unable to calculate the neonates' oxygenation indices as a measure of the severity of respiratory failure.[1,31] None of the neonates were treated with iNO or ECMO, as our unit did not offer these treatment modalities at the time.
Data showed that magnesium sulphate and sildenafil were used for pulmonary vasodilatation in 16.7% and 12.5% of neonates, respectively. This is similar to findings of other studies[6,32,33] and it has been shown that magnesium sulphate and sildenafil are safe and cost-effective vasodilators for the treatment of PPHN when iNO is not available.[3435]
Hyperventilation is no longer practised in the treatment of PPHN owing to associated neurological complications and the development of chronic lung disease.[u] Sodium bicarbonate infusion is also being used less frequently than before and in our study only 22.2% of neonates were treated accordingly. Similar findings (25%) were reported by Abdel et al.[6]In a multicentre study in the USA, Walsh-Sukys et al.[2] reported an overall alkali infusion rate of 75% prior to the use of iNO becoming the preferred treatment option.
Adequate cardiac output and improved perfusion or oxygenation can be achieved by volume expansion, inotropic support, or both.[10,11,14,36] However, inotropes should be used cautiously as they can also increase the pulmonary artery pressure and worsen the right-to-left shunt.[10] Just over half of the neonates included in our study (52.8%) required inotropic support. This was lower than the 84% of cases in which inotropic support was used in a multicentre study in the USA.[2]
PPHN is associated with a high mortality rate, especially in resource-limited settings.[4] Of the 72 neonates included in this study, 25 (34.7%) did not survive. Similar mortality rates, either directly or indirectly related to PPHN, were reported in previous studies across the world, e.g.: 48% at Chris Hani Baragwanath Academic Hospital[8] and 31% at Tygerberg Children's Hospital[7] in SA; 25% at Al-Minya University Hospital in Egypt;[6] 26.6% at the Children's Hospital Multan in Pakistan;[5] 32% at Hospital de Säo Joäo EPE in Portugal[9] and 27.6% at the Chang Gung Children's Hospital in Taiwan.[29] High PPHN mortality in resource-limited settings may be attributed to new therapies such as HFOV, iNO and ECMO not being readily available. ECMO specifically is considered a rescue therapy for neonates with severe PPHN and who are unresponsive to other treatment modalities; 40% of neonates with PPHN do not respond to the combination of HFOV and iNO and require ECMO as a last resort.[3,10,13]
Of the 25 nonsurvivors in this study, 62.5% died within 24 hours of admission. Univariate analysis showed that the characteristics of survivors and non-survivors were similar, except for the need for inotropic support. As the use of inotropes is associated with a poor outcome, the availability of iNO as a treatment option might have reduced the mortality among this sample.
Study limitations
Owing to its retrospective design, this study had some limitations. There were some missing data regarding infant characteristics. It was not possible to calculate the oxygenation indices as there were no ventilation parameters available. The severity of respiratory failure could therefore not be determined.
Echocardiography was performed on only a few neonates. The incidence of PPHN may therefore have been underestimated, as diagnosis was based mainly on clinical observation.
Conclusion
In this study, MAS was found to be the most common underlying cause of PPHN. Infant characteristics were similar between survivors and non-survivors. Magnesium sulphate and sildenafil were the only pulmonary vasodilators used. There was a considerable mortality rate (34.7%) and the need for inotropic support was the only factor associated with poor outcome.
Reducing MAS incidence may be a cost-effective measure in mitigating PPHN. MAS incidence could be reduced by improving antenatal and intrapartum obstetric care, reducing postdate deliveries, proper monitoring of at-risk pregnancies, offering adequate neonatal resuscitation, using surfactant replacement therapy and initiating assisted ventilation for depressed neonates with MAS early on.
As ECMO therapy is expensive and labour intensive and thus currently inaccessible in our setting, serious consideration should be given to introducing iNO to reduce the PPHN-associated mortality rate.
Acknowledgements. None.
Author contributions. IH was the primary researcher and conducted this project for his MMed degree. DB supervised the project, assisting with formulation of research question, data analysis, review of manuscript drafts and submission of final manuscript
Funding. None.
Conflicts of interest. None.
References
1. Lakshminrushimha S, Kumar VH. Diseases of pulmonary circulation. In: Fuhrman BP, Zimmerman JJ, eds. Pediatric Critical Care. 4th ed. Philadelphia: Elsevier, 2011:638-645. [ Links ]
2. Walsh-Sukys MC, Tyson JE, Wright LL, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: Practice variation and outcomes. Pediatrics 2000;105(1 Pt 1):14-20. https://doi.org/10.1542/peds.105.L14 [ Links ]
3. D'cunha C, Sankaran K. Persistent fetal circulation. Paediatr Child Health 2001;6(10):744-750. https://doi.org/10.1093/pch/6.10.744 [ Links ]
4. Agrawal A, Agrawal R. Persistent pulmonary hypertension of the newborn: Recent advances in the management. Int J Clin Pediatr 2013;2(1):1-11. https://doi.org/10.4021/ijcp79w [ Links ]
5. Razzaq A, Quddusi AI, Nizami N. Risk factors and mortality among newborns with persistent pulmonary hypertension. Pak J Med Sci 2013;29(5):1099-1104. https://doi.org/10.12669/pjms.295.3728 [ Links ]
6. Abdel Mohsen AH, Amin AS. Risk factors and outcomes of persistent pulmonary hypertension of the newborn in neonatal intensive care unit of Al-Minya University Hospital in Egypt. J Clin Neonatol 2013;2(2):78-82. https://doi.org/10.4103/2249-4847.116406 [ Links ]
7. Smith J, Kirsten GF. Persistent pulmonary hypertension of the neonate in a developing country - does extracorporeal membrane oxygenation have a role to play? S Afr Med J 1993;83:742-745. [ Links ]
8. Velaphi S, van Kwawegen AV. Meconium aspiration syndrome requiring assisted ventilation: Perspective in a setting with limited resources. J Perinato 2008;28(Suppl 3):S36-S42. https://doi.org/10.1038/jp.2008.155 [ Links ]
9. Rocha G, Baptista MJ, Guimaraes H. Persistent pulmonary hypertension of non cardiac cause in a neonatal intensive care unit. Pulm Med 2012;10:818971. https://doi.org/10.1155/2012/818971 [ Links ]
10. Nair J, Lakshminrusimha S. Update on PPHN: Mechanisms and treatment. Semin Perinatol 2014;38(2):78-91. https://doi.org/10.1053/j.semperi.2013.11.004 [ Links ]
11. Konduri GG, Kim UO. Advances in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatr Clin N Am 2009;56:579-600. https://doi.org/10.1016/j.pcl.2009.04.004 [ Links ]
12. Oishi P, Datar SA, Fineman JR. Advances in the management of pediatric pulmonary hypertension. Respir Care 2011;56(9):1314-1339. https://doi.org/10.4187/respcare.01297 [ Links ]
13. Teixeira-Mendonc C, Henriques-Coelhob T. Pathophysiology of pulmonary hypertension in newborns: Therapeutic indications. Rev Port Cardiol 2013;32(12):1005-1012. https://doi.org/10.1016/j.repce.2013.06.026 [ Links ]
14. Abman SH. Recent advances in the pathogenesis and treatment of persistent pulmonary hypertension of the newborn. Neonatology 2007;91:283-290. https://doi.org/10.1159/000101343 [ Links ]
15. Bendapudi P, Barr S. Diagnosis and management of pulmonary hypertension of the newborn. Paediatr Child Healt 2013;24(1):12-16. https://doi.org/10.1016/j.paed.2013.05.021 [ Links ]
16. Abu-Osba YK, Galal O, Manasra K, Rejjal A. Treatment of severe persistent pulmonary hypertension of the newborn with magnesium sulphate. Arch Dis Child 1992;67(1 Spec No):31-35. https://doi.org/10.1136/adc.67.1_spec_no.31 [ Links ]
17. Daffa SH, Milaat WA. Role of magnesium sulphate in treatment of severe persistent pulmonary hypertension of the neoborn. Saudi Med J. 2002;23(10):1266-1269. [ Links ]
18. Porta NFM, Steinhorn RH. Pulmonary vasodilator therapy in the NICU: Inhaled nitric oxide, sildenafil, and other pulmonary vasodilating agents. Clin Perinatol 2012;39(1):149-164. https://doi.org/10.1016/j.clp.2011.12.006 [ Links ]
19. Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in Neonates with persistent pulmonary hypertension of the newborn: A pilot randomized blinded study. Pediatrics 2006;117(4):1077-1083. https://doi.org/10.1542/peds.2005-0523 [ Links ]
20. Ichiba S, Bartlett RH. Current status of extracorporeal membrane oxygenation for severe respiratory failure. Artif Organs 1996;20(2):120-123. https://doi.org/10.1111/j.1525-1594.1996.tb00712.x [ Links ]
21. Maslach-Hubbard A, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: History, development and current status. World J Crit Care Med 2013;2(4):29-39. https://doi.org/10.5492/wjccm.v2.i4.29 [ Links ]
22. Lazar DA, Cass DL, Olutoye OO, et al. The use of ECMO for persistent pulmonary hypertension of the newborn: A decade of experience. J Surg Res 2012;177(2):263-267. https://doi.org/10.1016/j.jss.2011.11.487 [ Links ]
23. Lewandowski K. Extracorporeal membrane oxygenation for severe acute respiratory failure. Crit Care 2000;4(3):156-168. https://doi.org/10.1186/cc689 [ Links ]
24. Xu H, Wei S, Fraser WD. Obstetric approaches to the prevention of meconium aspiration syndrome. J Perinatol 2008;28(Suppl 3):S14-S18. https://doi.org/10.1038/jp.2008.145 [ Links ]
25. Whitfield JM, Charsha DS, Chiruvolu A. Prevention of meconium aspiration syndrome: An update and the Baylor experience. Proc (Bayl Univ Med Cent) 2009;22(2):128-131. https://doi.org/10.1080/08998280.2009.11928491 [ Links ]
26. Alano MA, Ngougmna E, Ostrea EM Jr, Konduri GG. Analysis of nonsteroidal antiinflammatory drugs in meconium and its relation to persistent pulmonary hypertension of the newborn. Pediatrics 2001;107(3):519-523. https://doi.org/10.1542/peds.107.3.519 [ Links ]
27. Hernandez-Diaz S, van Marter LJ, Werler MM, Louik C, Mitchell AA. Risk factors for persistent pulmonary hypertension of the newborn. Pediatrics 2007;120(2):e272-282. https://doi.org/10.1542/peds.2006-3037 [ Links ]
28. Roofthooft MTR, Elema A, Bergman KA, Berger RMF. Patient characteristics in persistent pulmonary hypertension of the newborn. Pulm Med 2011;2011:85815. https://doi.org/10.1155/2011/858154 [ Links ]
29. Hsieh WS, Yang PH, Fu RH. Persistent pulmonary hypertension of the newborn: Experience in a single institution. Acta Paediatr Taiwan 2001;42(2):94-100. [ Links ]
30. Konduri GG. New approaches for persistent pulmonary hypertension of newborn. Clin Perinatol 2004;31:591-611. https://doi.org/10.1016/j.clp.2004.04.001 [ Links ]
31. Steinhorn RH. Neonatal pulmonary hypertension. Pediatr Crit Care Med 2010;11:(2 Suppl):S79-S84. https://doi.org/10.1097/PCC.0b013e3181c76cdc [ Links ]
32. Mohamed S, Matthews T, Corcoran D, Clarke T. Magnesium sulfate improves the outcome in persistent pulmonary hypertension of the newborn. Sudanese Journal of Paediatrics 2007;8:102-113. [ Links ]
33. Engelbrecht AL. Sildenafil in the management of neonates with PPHN: A rural regional hospital experience. S Afr J Child Health 2008;2:166-169. [ Links ]
34. Tolsa JF, Cotting J, Sekarski N, Payot M, Micheli JL, Calame A. Magnesium sulphate as an alternative and safe treatment for severe persistent pulmonary hypertension of the newborn. Arch Dis Child Fetal Neonatal Ed 1995;72(3):F184-F187. https://doi.org/10.1136/fn.72.3.f184 [ Links ]
35. Dehdashtian M, Tebatebae K. Magnesium sulphate as a safe treatment for persistent pulmonary hypertension of newborn resistant to mechanical hyperventilation. Pak J Med Sci 2007;23(5):693-697. [ Links ]
36. Sharma M, Mohan KR, Narayan S, Chauhan L. Persistent pulmonary hypertension of the newborn: A review. Med J Armed Forces India 2011;67:348-353. https://doi.org/10.1016%2FS0377-1237(11)60082-8 [ Links ]
 Correspondence:
Correspondence:
I Harerimana
harinosa@gmail.com
Accepted 21 December 2017














