Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.11 no.4 Pretoria Dez. 2017
http://dx.doi.org/10.7196/sajch.2017.v11i4.1327
RESEARCH
doi:10.7196/sajch.2017.v11i4.1327
Placental malaria and neonatal anti-tetanus antibody status: Any association?
M F BashirI; H A ElechiII; G M AshirII; A I RabasaII; A B MusaIII; R T AkuhwaII; A G FaroukIV
IMBBS, FWACP (Paed); Department of Paediatrics, Abubakar Tafawa Balewa University Teaching Hospital, Bauchi, Nigeria
IIMBBS, FWACP (Paed); Department of Paediatrics, College of Medical Sciences, University of Maiduguri, Nigeria
IIMBBS, FWACP (Paed); Department of Paediatrics, College of Medical Sciences, University of Maiduguri, Nigeria
IIMBBS, FWACP (Paed); Department of Paediatrics, College of Medical Sciences, University of Maiduguri, Nigeria
IIIMSc; Department of Medical Laboratory Science, College of Medical Sciences, University of Maiduguri, Nigeria
IVMBBS, FMC Paed; Department of Paediatrics, College of Medical Sciences, University of Maiduguri, Nigeria
ABSTRACT
BACKGROUND. Neonatal tetanus (NT) has long remained an important cause of neonatal morbidity and mortality in the tropics, where it coexists with a high prevalence of placental malaria. The current strategy for the control of NT involves stimulating the production of a protective level of an anti-tetanus antibody in the mother, through tetanus toxoid immunisation, and transferring it through the placenta to the fetus. Placental malaria is known to alter the morphology and functions of the placenta, but the results of studies on the effect of the transfer of the anti-tetanus antibody, specifically, remain inconclusive.
OBJECTIVE. To study the influence of placental malaria on the transplacental transfer of anti-tetanus antibodies among mother-infant pairs at the University of Maiduguri Teaching Hospital in north-eastern Nigeria.
METHOD. Maternal and cord-blood samples were collected from 162 mother-infant pairs, and analysed for anti-tetanus antibody levels using the enzyme-linked immunosorbent assay technique. Placental biopsies were also taken from each mother-infant pair, and placental malaria diagnosed histologically.
RESULTS. A total of 71.6% (n=116) of the 162 mother-infant pairs were positive for placental malaria, out ofwhom 50.9% (n=59) had chronic-active, 37.9% (n=44) acute and 11.2% (n=13) past placental malaria. In addition, 25.3% (n=41) babies were classified as seronegative for tetanus antibodies, of whom 72.7% (n=32) were delivered to mothers who were positive for placental malaria. A total of 34.5% (n=56) mother-infant pairs had poor placental transfer for tetanus antibodies, as signified by a cord-maternal ratio of <1.0 antibodies; of these, 24.7% (n=40) were positive for placental malaria. There was a statistically significant association between type of placental malaria and serostatus (p=0.0009), and efficiency of placental transfer (p=0.0340). Mothers with chronic-active malaria were 7.4 times more likely to deliver a seronegative infant compared with mothers with acute malaria (p=0.0002; odds ratio (OR) 7.353; 95% confidence interval (CI) 2.327 - 23.234). Similarly, maternal-infant pairs with chronic-active malaria were 2.9 times more likely to have inefficient placental transfer (p=0.0221; OR 2.859; 95% CI 1.200 - 6.859).
CONCLUSION. Placental malaria has remained a very common medical condition in Maiduguri among pregnant women, and may partly account for the high level of neonatal tetanus prevalent in the area.
Globally, neonatal tetanus accounts for 7% of neonatal mortality,[1] but in Nigeria, one of the 27 countries that account for 90% of the global burden of the disease, it accounts for up to 20%.[1-4] Reports from different centres across the six geopolitical zones of Nigeria revealed very high case-fatality rates, with some approaching 100%.[5-11] Strengthening routine immunisation against tetanus for pregnant mothers using tetanus toxoid (TT) is considered the single most effective strategy, independent of other interventions, in eliminating neonatal tetanus. [12] The effectiveness of this strategy depends on the integrity of the placenta, which is the only medium of exchange between the mother and the fetus.
Placental transfer of the immunoglobulin IgG is a facilitated process that appears to be mediated by a specific fetal Fc receptor on the syncytiotrophoblastic cells.[13] Binding of IgG to this receptor leads to endocytosis, and it is subsequently actively transported across the villous stroma, basement membrane and, finally, through the endothelial cells of the fetal villi vessels.[14] The process becomes more efficient in late gestation; in one study, fetal concentrations of IgG immunoglobulin approximated the maternal levels at 38 weeks' gestation, and continued to increase until birth, reaching more than twice the maternal levels by delivery.[15] Certain prevailing factors in the tropics, however, have been shown to interfere with the transplacental transfer of IgGs: hypergammaglobulinaemia, owing to endemic and recurrent infections in the tropics, inversely affects the transfer of specific IgG, owing to the saturation of the Fc receptor;[16] HIV infection has also been shown to impair the ability of the mother to produce and transfer adequate IgGs to her fetus.[17]Placental malaria can produce pathological changes in the placenta, such as thickening of the basement membrane, inflammatory cell infiltrates, villitis and microinfarct owing to clumping of parasitised red blood cells and the occlusion of microvasculatures.[18] These changes could lead to a reduction in blood flow to the intervillous spaces, and alteration in the structure of the placental barrier, thereby impacting negatively on the transplacental transfer of molecules. In Nigeria, placental malaria remains highly endemic,[19,20] and has been shown to impair the transplacental transfer of maternal IgG needed to fight against common childhood infectious diseases such as tetanus,[21,22] measles[23] and the Epstein-Barr virus.[24]
However, there are conflicting reports regarding the effect of placental malaria on the efficiency of the transplacental transfer of the anti-tetanus antibody, and therefore on the cord-blood concentrations. In a malarial area of Papua New Guinea,[21] the cord-maternal ratio (CMR) of the anti-tetanus antibody among pregnant women with heavy placental malarial infections was considerably lower (0.18) than that among women without such infections (0.83). Approximately 10% of babies born to mothers whose placenta were heavily infected with Plasmodium falciparum failed to acquire protective levels of the anti-tetanus antibody, despite adequate maternal antibody concentrations.[21] Additionally, Cumberland et al.l22]reported that in Kenya, maternal and neonatal anti-tetanus antibody serum levels, as well as transplacental transfer, were reduced when women had chronic-active or past placental malaria. By contrast, studies in Malawi[25] and The Gambia[23] did not find any effect of placental malaria on anti-tetanus antibody serum levels or transplacental transfer. However, these conflicting findings could be accounted for by differences in methodology: in the Malawian study,[25-in addition to a smaller sample size, thick and thin films from placental blood smear were used, rather than placental histology, which assessed only the presence or absence of parasites, and therefore excluded past placental malaria, one of the groups with a significant effect on transplacental transfer in the Kenyan study.[22] Furthermore, those with placental malaria in both the Malawian[25] and Gambian[23] studies were not further subclassified into acute and chronic. This may be an important distinction, as demonstrated in the Kenyan study,[22] where acute placental malaria had no significant effect on transplacental transfer of the antibody, in contrast to active chronic and past placental malaria. Therefore, the cases of acute malaria in both studies[23,25] might have influenced the stated results. Similarly, neither of the two studies[23,25] subclassified those with placental malaria based on the degree of parasitisation, which was identified by Brair et al.[21]as an important factor in Papua New Guinea.
These conflicting findings, coupled with the high prevalence of neonatal tetanus and placental malaria in Nigeria, make it imperative to ascertain the effect of the latter on the former if the scourge is to be overcome. Therefore, we aim to determine the effect of placental malaria on cord serum levels of the anti-tetanus antibody, as well as its effect on the transplacental transfer of this antibody.
Methods
Study area
The study was carried out at the labour ward of the University of Maiduguri Teaching Hospital (UMTH), Maiduguri, Borno State, Nigeria. UMTH is a tertiary healthcare centre located in northeastern Nigeria, and a centre of excellence for infectious diseases research and immunology. It also serves as a referral site for the six north-eastern states and the neighbouring countries of Chad, Cameroon and Niger.
Ethical considerations
The protocol of this hospital-based cross-sectional descriptive study was reviewed and approved by the Research and Ethics Review Committee of UMTH (ref. no. ADM/TH.75/Vol.II). Signed or thumb-printed informed consent was obtained from each mother. Confidentiality was maintained, and mothers were informed of the outcome of the investigations.
Sample size determination
The minimum sample size was determined using Cochran's sample size formula for categorical data,[26] at an a level of 0.05 and a standard normal deviate of 1.96, corresponding to a 95% confidence interval (CI). The proportion of newborns with protective levels of tetanus IgG antibody (seropositives) was selected using the method from a previous study in Jos by Adabara et al.,[27]at 88%. The sample size for the study, therefore, was 162 mother-infant pairs.
Data collection
Mother-infant pairs were enrolled using the systematic random sampling method; the first of every three mother-infant pairs were selected at the labour ward from the first day of data collection, to ensure an even spread over the period of study. Mothers who had had stillbirths, or had a history of anti-tetanus serum (ATS) administration or blood transfusion within the preceding 4 weeks to delivery, were excluded. Also excluded were mothers with diabetes mellitus, hypertension, eclampsia or pre-eclampsia, as a result of the known effects of these conditions on placental function.[28-Upon enrolment, a questionnaire on sociodemographic variables was completed for each mother-infant pair. This included biodata, pregnancy and antenatal history and gestational age, as well as indicators of socioeconomic status such as the occupations and educational levels of both parents. A history of TT vaccination during the current pregnancy, verifiable from the patient's antenatal clinic records, was obtained where possible, including the number of doses and their interval, as well as the number of TT doses received before the index pregnancy. Gestational age at delivery was determined by ultrasound scan, where available, or by date using the last menstrual period, and confirmed by the New Ballard score[29] of the baby after delivery. Socioeconomic status was assigned using Oyedeji's[30] model. Birth weight was measured using a digital weighing scale; babies weighing <2.50 kg were classified as low birth weight.[31] All weights were measured to the nearest 0.01 kg.
Sample collection and analysis
Maternal venous blood (2 mL) was collected from a peripheral vein following an aseptic procedure. Additionally, 2 mL of cord blood from the placental end of the cord, after early clamping of the cord, was also collected, and stored in properly labelled sterile plain bottles. Sera were separated after centrifuging these blood samples at 5 000 rpm for 5 minutes. Aliquots of serum samples were stored at -200C until analysis. The serums from each mother-infant pair were matched, and assayed for tetanus IgG antibodies by the enzyme-linked immunosorbent assay (ELISA) technique (Demeditec Diagnostics, Germany). The optical density was measured at 450 nm (OD450) in an ELISA microplate reader. Standard curves were drawn for each plate, and optical densities of the test-serum dilutions falling within the linear part of the curve were extrapolated. The results were expressed as IU/mL. Antibody levels below 0.1 IU/mL were classified as seronegative, in accordance with World Health Organization (WHO) recommendations. [32]
A full-thickness placental biopsy specimen was obtained from a healthy pericentric area, and fixed in 10% neutral buffered formal saline for 24 hours. It was then sent to the histopathology laboratory, where subsequent processing was carried out. Representative samples were taken from fixed placental tissue, and processed using the Shandon automatic tissue-processing machine (Shandon Southern Instruments Ltd., UK) over a period of 24 hours. The tissue was then embedded using paraffin wax, and sections were cut at 4 μηι, mounted on a slide, dewaxed using a hot plate and cleaned in xylene, before hydrating in descending grades of ethanol and, finally, washed in water and stained with Giemsa stain (1:10 dilution) for 30 minutes. Finally, under a coverslip, it was examined under a x40 lens of a 4 χ 4 Olympus electric microscope (Olympus Optical Co., Ltd., Japan). The presence of malaria parasites and/or pigment was considered positive for placental malaria. Those classified as positive were further subclassified into acute placental malaria, chronic-active malaria and past malaria, based on the presence of malaria parasites alone, malaria parasites and pigment, and pigment alone, respectively.
Data analysis
Data obtained were entered into a computer that generated a computerised data base. Analysis was done using SPSS version 16.0 (SPSS Inc., USA). Tables were used for data presentation as appropriate. Geometric means and standard deviations (SDs) of antitetanus antibody titres were determined. Frequencies of anti-tetanus antibody serostatus (seropositives and seronegatives) were compared using Fisher's exact test. A _p-value of <0.05 was considered significant.
Results
A total of 162 mother-infant pairs were enrolled. The mean (SD) age of the mothers was 27.2 (6.0) years. Most (74.7%; n=121) were multipara, and belonged to the low socioeconomic class, 81.5% (n=132). A total of 85.2 (n=138) mothers had had at least two TT vaccinations during the current pregnancy, and 71.6% (n=116) of them were positive for placental malaria (Table 1). Out of those with placental malaria, 38% (n=44) had acute malaria, 59 (51%) chronic -active malaria while 11% (n=13) had past malaria.
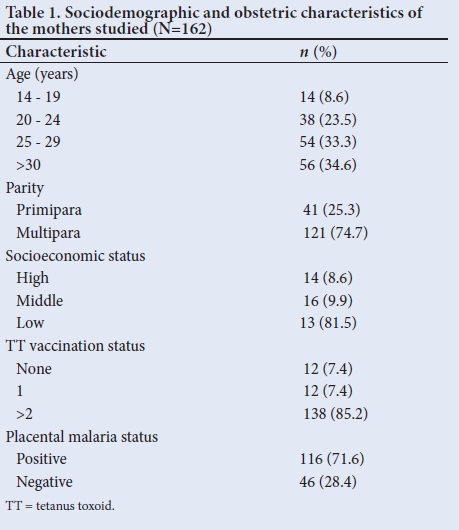
Of the 162 babies enrolled, 51.9% (n=84) were male and 48.1% (n=78) were female, giving a male to female ratio (M:F) of 1.08:1. A total of 92% (n=149) were term and the remaining 8% (n=13) were preterm. None of the babies was post term. Low birth weight was observed in 9 5.6% (n=9) of babies, while the remaining 94.4% (n=153) were of normal birth weight.
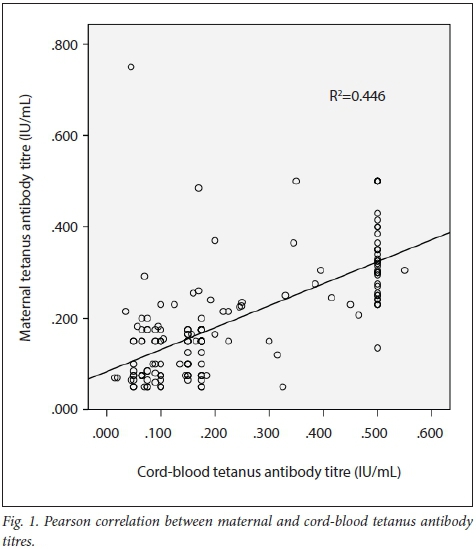
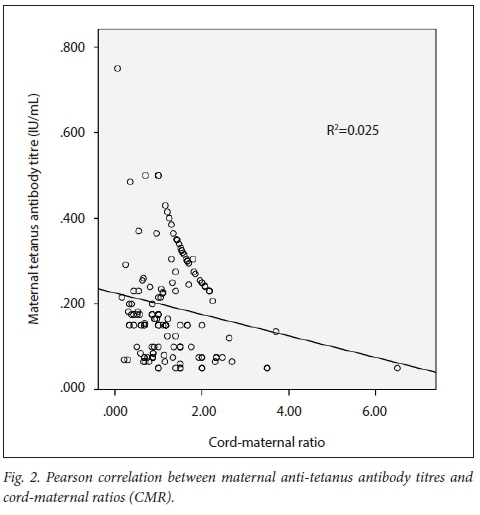
The mean (SD) anti-tetanus antibody titre of the mothers and their babies was 0.160 (0.122) IU/mL and 0.230 (0.170) IU/mL, respectively, giving an overall CMR of 1.437. A total of 76.5% (n=124) of mothers and 74.4% (n=121) of babies were seropositive for tetanus antibodies (titre of >0.1 IU/mL). There was a strong positive correlation between maternal and cord-blood levels of tetanus antibodies (r=0.668; p<0.001), with a shared variance of 44.6% (Fig. 1). There was a weak but significant negative correlation between maternal anti-tetanus antibody levels and CMRs (r=-0.158; p=0.045) with a shared variance of only 2% (Fig. 2).
A total of 25.3% (n=41) of babies were classified as seronegative for tetanus antibodies (titre of <0.1 IU/mL), out of whom 72.7% (n=32) were delivered to mothers who were positive for placental malaria. Similarly, 34.5% (n=56) mother-infant pairs had poor placental transfer (CMR <1) for tetanus antibodies, out of whom 24.7% (n=40) were positive for placental malaria. However, placental malaria did not show a significant association with either the tetanus serostatus of the babies (p=0.3233) or with the efficiency of placental transfer of tetanus antibodies (p=1.000).
Of the 32 seronegative infants born to placental-malaria positive mothers, 78.1% (n=25) of the mothers had chronic-active malaria, while 12.5% (n=4) and 9.4% (n=3) had acute and past malaria, respectively. Similarly, of the 40 maternal-infant pairs with CMR <1 and placental malaria positive, 67.5% (n=20), 25.0% (n=10) and 7.5% (n=3) had chronic-active, acute and past malaria respectively. There was a statistically significant association between type of placental malaria and serostatus (p=0.0009) and with the efficiency of placental transfer (p=0.0340). Mothers with chronic-active malaria were 7.4 times more likely to deliver a seronegative infant than mothers with acute malaria (odds ratio (OR) 7.353; 95% CI 2.327 - 23.234; p=0.0002) (Table 2) Similarly, maternal-infant pairs with chronic- active malaria were 2.9 times more likely to have inefficient placental transfer (OR 2.859; 95% CI 1.200 - 6.859; p=0.0221) than those with acute malaria (Table 3).
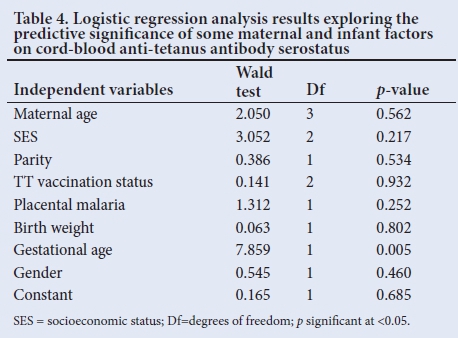
Among the remaining maternal and infant factors studied, only gestational age at birth showed a statistically significant association with cord-blood serostatus (£=0.004) as well as CMR (£=0.002). The preterms were 5.624 times more likely to be seronegative (OR 5.624; CI 1.961 - 28.41) and 7.464 times more likely to have inefficient transfer of anti-tetanus antibody (OR 7.464; CI 1.724 - 18.35) compared to term neonates.
Logistic regression analysis showed that only an infant's gestational age could significantly predict the anti-tetanus antibody serostatus, even after controlling for all other factors in the model (Table 4).
Discussion
The 71.6% prevalence of placental malaria among the mothers in this study was more than twice that reported by Bako et al.[19](33.9%) from the same centre. It is also higher than the 57.6% reported by Ibhanesebhor and Okolo[20] in Benin, Nigeria, as well as the 51.1% reported in The Gambia by Okoko et al.[21]However, this higher prevalence observed in this study when compared with these earlier studies may be accounted for by the difference in the criteria used for the diagnosis of placental malaria. While the other studies used only the presence of the malaria parasite as the diagnostic criterion, this study used the malaria parasite and/or malaria pigment, so that past or chronic malaria, which are more likely to be associated with structural and functional changes in placenta than acute malaria, could also be diagnosed.[33]
Similarly to earlier studies,[22,27,34] in both mothers and babies, the overall geometric mean titres of anti-tetanus antibodies were within the protective range (>0.1 IU/mL), with a correspondingly high proportion of anti-tetanus antibody seropositivity. Cumberland et al.[22]in 2007 reporting from Kenya found the geometric mean titres of anti-tetanus antibody of both the mothers and their babies to be within the protective range. Similarly, Hood et al.[34]found the geometric mean concentration of anti-tetanus antibodies in Nigerian mothers and their infants to be within the protective range. The
proportion of seropositive mothers and babies were similarly very high in these studies by Hood et al.,[34] Adabara et al.[21](both from Nigeria) and Cumberland et al.[22] from Kenya. However, the findings from this study and the earlier ones[22,27,34] may not be a true reflection of the anti-tetanus antibody serostatus in the general population, because women who deliver in hospital are more likely to have received the TT vaccination than women who deliver at home.'351 This is further supported by the higher incidence of neonatal tetanus among home-delivered infants,[36] indicating a lower or non-protective anti-tetanus antibody level. It is known that in Nigeria and other developing countries, the proportion of pregnant women not enrolling for antenatal care has continued to remain high, with an attendant high proportion of home deliveries.[37]
The mean CMR found in this study was higher than that in most previous studies, such as in Ibadan, Nigeria,[34] Kenya,[22] Libreville, Gabon,[38] Thailand[39] and India.[40] While it was found to be >1.0 in this study, signifying efficient placental transfer, the others reported <1.0, pointing to the earlier held view of poor placental transfer of tetanus antibodies in African mothers. However, a further limitation, apart from the small sample size in the Ibadan study, lay in the fact that most of the studies used an in vitro technique to determine antitetanus antibody levels that measures both IgG and IgM antibodies, resulting in misleadingly high maternal titres, and therefore low CMRs, compared with the standard ELISA (in vivo) used in this study, which measures only IgG.
A highly significant positive correlation was observed between the maternal and baby tetanus antibody levels, affirming the direct relationship between the two, with the later dependent on the former. A similar significant positive correlation was reported by Adabara et al.[27] in Nigeria. However, in our study, a significant but weak negative correlation was found to exist between maternal tetanus antibody levels and CMR, with only about 2% of its variance being attributable to changes in maternal tetanus antibody levels. The reason for the decreasing efficiency of the transplacental transfer of anti-tetanus antibody with increasing maternal levels of the antibody is not clear. It may be that mothers with high tetanus antibody levels might generally be hypergammaglobinaemic. It is known that this condition is associated with reduced placental transfer of tetanus antibodies, as previously reported.[23,41,42] High maternal IgG has been found to be common in Africa.[23,42] This is owing to the fact that the IgG binding site on the FcRn receptor can become saturated. Therefore, the amount of IgG transmitted depends on the number of cell surface receptors, because unbound IgG molecules are digested by lysosomal enzymes inside the vesicles.[43] Michaux et al.[38] reported that total IgG concentrations in cord sera tend to be lower than in the mothers when total IgG levels in maternal serum reached 15 g/L.
Similarly to the observations in Malawi by de Moreas-Pinto et al.[25] and Okoko et al.[23] in The Gambia, this study found no significant association between placental malaria and materno-fetal transfer of tetanus antibodies, or cord-blood tetanus antibody levels. However, this finding seems to be the result of the cofounding effects of the various types of placental malaria on one another. This is evident from the significant association, demonstrated in this study, between the types of placental malaria and cord-blood levels (serostatus) of the tetanus antibody, as well as the efficiency of tetanus antibody transfer. As Cumberland et al.[22]also found, chronic-active placental malaria was significantly associated with reduced transplacental transfer and cord-blood levels of tetanus antibody in this study. The reason for the difference between chronic-active malaria and the other types of placental malaria in this study is not clear, but may be the result of differences in the extent and type of changes to the placental membrane. However, in contrast to this study, Cumberland et al.22also demonstrated that past placental malaria was significantly associated with reduced cord-blood levels, and poor tetanus antibody transfer. The reason for this contrasting finding remains unclear, but this study might not have demonstrated the effect of past placental malaria owing to the small number of mothers involved who had past placental malaria. It could also be that active inflammation and past damage to the placenta act synergistically to cause the observed effect of chronic-active malaria, but neither individually would have had a significant effect on cord-blood levels and the efficiency of tetanus antibody transfer, as in this study.
Conclusion and recommendations
Placental malaria remains a very common medical condition in Maiduguri among pregnant women, and may partly account for the high level of neonatal tetanus prevalent in the area. Prompt treatment of cases of malaria in pregnancy, as well as scaling up of the strategy of intermittent preventive treatment of malaria in pregnancy might go a long way towards reducing the scourge of neonatal tetanus.
Acknowledgements. We wish to acknowledge Prof. A Mayun and Dr A Bukar, both of the Department of Histopathology, as well Mr D Bukbuk and Mr S Joshua of the Immunology Department, UMTH, for their expert contribution in viewing the slides and running the ELISA.
Author contributions. MFB conceived the idea, prepared a proposal for the research and collected and analysed the data. HAE participated in data analysis, and prepared the manuscript for publication. GMA corrected the proposal and manuscript. AIR supervised and made corrections in all stages of the study. ABM preserved and processed the placental tissue for viewing while RTA corrected the proposal for the study. AGF read through and corrected the proposal and manuscript.
Funding. None.
Conflicts of interest. None.
References
1. Lawan JE, Cousens S, Zupan J. 4 million neonatal deaths: When? Where? Why? Lancet 2005;365(9462):891-900. https://doi.org/10.1016/s0140-6736(05)71048-5 [ Links ]
2. United Nations International Children's Emergency Fund, World Health Organization, United Nations Population Fund. Maternal and Neonatal Tetanus Elimination by 2005: Strategies for Achieving and Maintaining Elimination. New York: UNICEF, 2000. [ Links ]
3. Babaniyi O, Parakoyi B. Cluster survey for poliomyelitis and neonatal tetanus in Ilorin, Nigeria. Int J Epidemiol 1991;20(2):515-520. https://doi.org/10.1093/ije/20.2.515 [ Links ]
4. Eregie CO. Uvulectomy as an epidemiological factor in neonatal tetanus mortality: Observation from a cluster survey. West Afr J Med 1994;13(1):56-8. [ Links ]
5. World Health Organization. Incidence of neonatal tetanus in Kano State, Nigeria, 2006. Wkly Epidemiol Rec 2006;81(46):433-440. [ Links ]
6. Eregie CO, Ofovwe G. Cluster survey on neonatal mortality in Nigeria: Observation of some clinical aspects. J Trop Pediatr 1993;39(6):372-373. [ Links ]
7. Omoigberale AI, Abiodun PO. Upsurge in neonatal tetanus in Benin City, Nigeria. East Afr Med J 2005;82(2):98-102. [ Links ]
8. Oruamabo RS, Igbagiri FP. Neonatal tetanus in Port Harcourt. Afr J Med Med Sci 1996;25(3):265-268. [ Links ]
9. Asekun-Olarinmoye EO, Lawoyin TO, Onadeko MO. Risk factors associated with neonatal tetanus in Ibadan, Nigeria - a revisit. Afr J Med Med Sci 2003;32(3):275-278. [ Links ]
10. Owa JA, Makinde OO. Neonatal tetanus in babies of women immunized with TT during pregnancy. Trop Doct 1990;20(4):156-157. [ Links ]
11. Osuhor PC. Neonatal tetanus in Zaria, northern Nigeria. Indian J Public Health 1983;27(1):32-37. [ Links ]
12. Gupta SD, Keyl PM. Effectiveness of prenatal TT immunization against neonatal tetanus in a rural area in India. Pediatr Infect Dis J 1998;17(4):316-321. [ Links ]
13. Chucri TM, Monteiro JM, Lima AR, Salvadori MLB, Kfoury JR, Miglino MA. A review of immune transfer by the placenta. J Reprod Immunol 2010;87(1-2):14-20. https://doi.org/10.1016/j.jri.2010.08.062 [ Links ]
14. Blackburn ST. The prenatal period and placental physiology. In: Blackburn S. Maternal, Fetal & Neonatal Physiology: A Clinical Perspective (4th ed.). Washington: Elselvier, 2013:61-114. [ Links ]
15. Pitcher-Willnot RW, Hindocha P, Wood CBS. The placental transfer of IgG subclasses in human pregnancy. Clin Exp Immunol 1980;41(2):303-308. [ Links ]
16. Gendel D, Richard-Lenoble D, Massamba MB, Picaud A, Francual C, Blot P. Placental transfer of tetanus antibodies and protection of the newborn. J Trop Pediatr 1990;36(6):279-282. https://doi.org/10.1093/tropej/36.6.279 [ Links ]
17. Bashir MF, Elechi HA, Ashir MG, et al. Neonatal tetanus immunity in Nigeria: The effect of HIV infection on serum levels and transplacental transfer of antibodies. J Trop Med 2016. https://doi.org/10.1155/2016/7439605 [ Links ]
18. Galbraight RM, Fox H, His B, Galbraight GMP, Bray RS, Faulk WP. The materno-fetal relationship in malaria: histological, ultrastructural and immunopathological studies of the placenta. Trans R Soc Trop Med Hyg 1980;74(1):61-72. https://doi.org/10.1016/0035-9203(80)90012-7 [ Links ]
19. Bako BG, Audu BM, Geidam AD, Kullima AA, Ashiru GM, Malah GM et al. Prevalence, risk factors and effects of placental malaria in the UMTH, Maiduguri, North-eastern Nigeria: A cross-sectional study. J Obstet Gynaecol 2009;29:(4):307-310. https://doi.org/10.1080/01443610902878783 [ Links ]
20. Ibhanesebhor SE, Okolo AA. Placental malaria and pregnancy outcome. Int J Gynaecol Obstet 1992;37(4):247-252. https://doi.org/10.10160020-7292(92)90324-C [ Links ]
21. Brair ME, Brabin BJ, Milligan P, Maxwell S, Hart CA. Reduced transfer of tetanus antibodies with placental malaria. Lancet 1994;343(8891):208-209. https://doi.org/10.1016/S0140-6736(94)90991-1 [ Links ]
22. Cumberland P, Shulman CE, Maple PAC, et al. Maternal HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis 2007;196(4):550-557. https://doi.org/10.1086/519845 [ Links ]
23. Okoko BJ, Wesumperuma LH, Ota MO, et al. Influence of placental malaria infection and maternal hypergammaglobulinaemia on materno-fetal transfer of measles and tetanus antibodies in a rural west African population. J Health Popul Nutr 2001;19(2):59-65. https://doi.org/10.1086/322808 [ Links ]
24. Ogalla S, Daud II, Asito AS, et al. Reduced transplacental transfer of a subset of Epstein-Barr virus-specific antibodies to neonates of mothers infected with Plasmodium falciparum malaria during pregnancy. Clin Vaccine Immunol 2015;22(11):1197-1205. https://doi.org/10.1128/cvi.00270-15 [ Links ]
25. De Moraes-Pinto MI, Verhoeff F, Chimsuku L, et al. Placental antibody transfer: Influence of maternal HIV infection and placental malaria. Arch Dis Child Fetal Neonatal Ed 1998;79(3):202-205. [ Links ]
26. Cochran WG. Sampling Techniques (3rd ed.). New York: John Wiley & Sons, 1977. [ Links ]
27. Adabara NU, Kandakai-Olukemi YT, Enenebeaku MN, Daru PH. Assessment of materno-fetal transfer of antitetanus immunoglobulin G in Jos University Teaching Hospital, Jos. Shiraz E-Med J 2010;11(2):1-8. [ Links ]
28. Vambergue A, Fajardy I. Consequences of gestational and pregestational diabetes on placental function and birth weight. World J Diabetes 2011;2(11):196-203. [ Links ]
29. Ballard JL, Khoury JC, Wedig K, et al. New Ballard Score, expanded to include extremely premature infants. J Pediatr 1991;119(3):417-423. https://doi.org/10.1016/S0022-3476(05)82056-6 [ Links ]
30. Oyedeji GA. Socioeconomic and cultural background of hospitalised children in Ilesha. Nig J Paediatr 1985;12(4):111-117. [ Links ]
31. Stoll BJ, Adams-Chapman I. The high-risk infant. In: Kliegman RM, Behrman RE, Jenson HB, Stanton BF, eds. Nelson Textbook of Pediatrics (18th ed.). Philadelphia: Saunders, 2007:97. [ Links ]
32. World Health Organization. Tetanus Vaccine: WHO Position Paper. Wkly Epidemiol Rec 2006;81(20):198-208. [ Links ]
33. Brabin BJ, Romagosa C, Abdelgalil S, et al. The sick placenta - the role of malaria. Placenta 2004;25(5):359-378. https://doi.org/10.1016/j.placenta.2003.10.019 [ Links ]
34. Hood N, Chan MC, Maxwell SM, Familusi JB, Hart CA. Placental transfer of tetanus toxoid antibodies in Nigerian mothers. Ann Trop Paediatr 1994;14(3):179-182. https://doi.org/10.1080/02724936.1994.11747714 [ Links ]
35. Cutts FT, Rodrigues LC, Colombo S, Bennett S. Evaluation of factors influencing vaccine uptake in Mozambique. Int J Epidemiol 1989;18(2):427-433. https://doi.org/10.1093/ije/18.2.427 [ Links ]
36. Alhaji MA, Bello MA, Elechi HA, Akuhwa RT, Bukar FL, Ibrahim HA. A review of neonatal tetanus in University of Maiduguri Teaching Hospital, North-eastern Nigeria. Niger Med J 2013;54(6):398-401. https://doi.org/10.4103/0300-1652.126294 [ Links ]
37. United Nations International Children's Emergency Fund. Statistics. In: At a Glance: Nigeria. New York: UNICEF, 2013. http://www.unicef.org/infobycountry/nigeria_statistics.html (accessed 10 December 2015). [ Links ]
38. Michaux JL, Heremans JF, Hitzig WH. Immunoglobulin levels in cord-blood serum of negroes and caucasians. Trop Geogr Med 1966;18(1):10-14. [ Links ]
39. Sangpetchsong V, Vichaikummart S, Vichitnant A, Podhipak A. Transfer rate of transplacental immunity to tetanus from nonimmunized and immunized mothers. Southeast Asian J Trop Med Public Health 1984;15(3):275-280. [ Links ]
40. Maselle SY. Maternal and fetal tetanus toxoid antibody levels following immunization in pregnancy. J Obstet Gynaecol East Cent Africa 1989;8(1):11-14. [ Links ]
41. Gendrel D, Richard-Lenoble D, Massamba MB, Picaud A, Francoual C, Blot P. Placental transfer of tetanus antibodies and protection of the newborn. J Trop Pediatr 1990;36(6): 279-282. https://doi.org/10.1093/tropej/36.6.279 [ Links ]
42. Hartter HK, Oyedele OI, Dietz K, Kreis S, Hoffman JP, Muller CP. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr Infect Dis J 2000;19(7):635-641. [ Links ]
43. Saji F, Koyama M, Matsuzaki N. Human placental Fc receptors. Placenta 1994;15(5):453-466. https://doi.org/10.1016/S0143-4004(05)80415-1 [ Links ]
 Correspondence:
Correspondence:
H A Elechi
h3elechi@unimaid.edu.ng
Accepted 12 September 2017














