Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.11 n.2 Pretoria Jun. 2017
http://dx.doi.org/10.7196/SAJCH.2017.v11i2.1244
REVIEW
Neonatal sepsis: Highlighting the principles of diagnosis and management
M CoetzeeI; N T MbowaneII; T W de WittIII
IMB ChB, DCH, FCPaed, MMed (Paed), Cert Neonatology (SA); Division of Neonatology, Department of Paediatrics and Child Health, School of Medicine, University of Pretoria, South Africa
IIMB ChB, FCPaed; Division of Neonatology, Department of Paediatrics and Child Health, School of Medicine, University of Pretoria, South Africa
IIIMB ChB, MMed (Paed), FCPaed, DTE; Division of Neonatology, Department of Paediatrics and Child Health, School of Medicine, University of Pretoria, South Africa
ABSTRACT
Neonatal sepsis is a clinical syndrome consisting of nonspecific symptoms and signs of infection, accompanied by a bacteraemia in the first 28 days of life. The risk of neonatal sepsis and death increases with decreasing birth weight and gestational age. South African data have reported the overall incidence of neonatal sepsis to be 8.5 - 10%, with late-onset sepsis accounting for most of these infections. The diagnosis of neonatal sepsis is not always straightforward, and the initiation and continuation of antimicrobials in these situations relies on good clinical judgment. The need for empirical antimicrobials is driven by the existence of risk factors for early-onset sepsis and clinical symptoms and signs of late-onset sepsis. Antimicrobial stewardship programmes should be in place to guide clinicians to either stop, change, or continue antimicrobials. Institution-specific knowledge of the most common pathogens and the antimicrobial susceptibility pattern is important to prevent the emergence of further antimicrobial resistance.
Neonatal sepsis is a clinical syndrome consisting of nonspecific symptoms and signs of infection accompanied by bacteraemia in the first 28 days of life.[1,2] Early-onset sepsis (EOS) presents within the first 72 hours of life, and late-onset sepsis (LOS) presents after 72 hours of life.[3-6] The nonspecific features of sepsis may include lethargy, poor feeding or feeding intolerance, irritability, temperature instability, brady- or tachycardia, glucose instability, poor perfusion, apnoea, and a bleeding tendency.[1,2]
EOS is primarily the result of intrapartum vertical transmission of bacteria from the mother to the neonate, either transplacentally or due to ascending infection from the genital tract.[2-4,6] LOS is the result of horizontal transmission of bacteria from the environment and healthcare providers' hands,[3,6] and has a peak incidence at between 15 and 17 days of life.[2]
Incidence and mortality
The risk of neonatal sepsis and death increases with decreasing birth weight[6] and gestational age,[4,7] with better outcomes in neonates that receive early empirical antimicrobial treatment.[3] However, ~95% of neonates initiated on empirical antimicrobials for suspected EOS were shown to have no laboratory evidence of infection.[7] Available South African (SA) data have reported the overall incidence of neonatal sepsis to be 8.5 - 10%, with LOS accounting for the majority of these infections (83.2 - 94.3%).[8-9] The mortality rate was found to vary between 24.2% and 40%, and 19.7% and 22.5% for EOS and LOS, respectively, with an overall mortality of 20.8 - 23%.[8,9] Death due to Gram-negative sepsis is more common (69.2 - 80%).[8,9]
Making the diagnosis of neonatal sepsis
The gold standard for confirming neonatal sepsis is a positive culture from a sterile site, including blood,[6,10] cerebrospinal fluid (CSF)[7] or urine.[11] Culture results may only become available after 48 - 72 hours, and initiation and continuation of antimicrobials in these situations often rely on good clinical judgement.
Blood investigations, including the full blood count and acute-phase reactants, such as C-reactive protein (CRP) and procalcitonin (PCT), are time-dependent and should be performed 6 - 12 hours after delivery to allow for an inflammatory response.[4,8] The total white cell count has a poor positive predictive value for neonatal sepsis and is not useful in making the diagnosis.[4,5] While neutropenia has a better specificity for neonatal sepsis, values are dependent on gestational age, time after birth, and delivery method.[4,5] The immature-to-total-neutrophil ratio (I:T ratio) has the best sensitivity of all the neutrophil indices, but the positive predictive value is only 25%.[4] The I:T ratio is best used to exclude neonatal sepsis, with a negative predictive value of 99%.[4] CRP values start to increase 6 - 8 hours after infection and peak at ~24 hours,[4] with only 35 - 65% of neonates having a raised CRP at the onset of illness.[3] Serial CRP values at 24 - 48 hours after the onset of sepsis have a better sensitivity and specificity when compared with a single CRP value.[3] Two consecutive CRP values of <10 mg/L, obtained 24 hours apart, have a negative predictive value of 99%.[3-5] PCT is slightly more sensitive than CRP, but with a lower specificity,[4] as it may increase physiologically in the first 24 hours post-delivery, and may increase in response to non-infectious conditions.[4,5] PCT values increase 2 hours after infection and peak ~12 hours later.[4] A PCT value of <0.1 ng/mL is considered normal in a neonate >72 hours old.[5]
At least 1 mL of blood should be collected for sterile blood cultures, with appropriate measures taken to reduce contamination. Suggested factors to differentiate between true infection and contamination include the identity of the organism, the number of positive cultures of the same organism, the time taken to flag positive, the quantity of bacterial growth, and the source or site of the culture.[10] The positive predictive value for true bacteraemia improves when multiple cultures (>2) grow the same organism,[12,13] with the presence of only one positive culture out of at least two being suggestive of contamination.[10] Cultures that flag positive after 48 - 72 hours are more likely to be contaminants.[10,12,13] Limited evidence exists for using the quantity of bacterial growth to differentiate true bacteraemia from contamination, and low colony counts in a high-risk population should not be dismissed as contamination.[10] Lastly, cultures taken from a vascular catheter may represent true bacteraemia, contamination, or catheter colonisation. To distinguish between these, it is recommended that cultures are taken from both the catheter and peripherally.[10]
Coagulase-negative staphylococci (CoNS), which are skin commensals and represent ~80% of contaminated blood cultures,[10] have recently emerged as pathogenic organisms causing LOS, especially in premature and very-low-birth-weight (VLBW) neonates.[2'5'6] To exclude contamination, most clinicians require at least 2 positive cultures of CoNS.[21 Another study has shown that <15 hours' time to positivity for CoNS had a positive predictive value of 84% for true bacteraemia.[10] CoNS is responsible for more than 50% of cases of LOS in developed countries, and 35 - 46.5% in developing countries, with a mortality rate of up to 10.2% in VLBW neonates.[2] Indwelling medical devices are the largest contributing factor for CoNS sepsis in VLBW infants.[2]
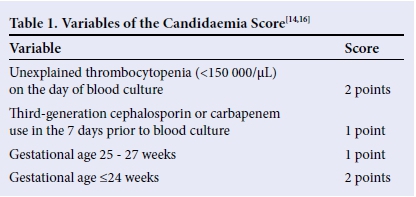
The sensitivity for diagnosing invasive fungal sepsis on blood culture remains poor (<50%).[14-15] Risk factors for fungal sepsis include extremely low birth weight (ELBW), gestation <28 weeks, previous exposure to antibiotics (third-generation cephalosporins or carbapenems), thrombocytopenia, central venous lines, mechanical ventilation, use of antacids (histamine-2 blockers or proton-pump inhibitors), use of total parenteral nutrition, delayed enteral feeding, and prolonged hospital stay (>7 days).[3,14,16] A clinical predictive model for candidaemia is available (Table 1),[14,16] with a combined score of 2 having a sensitivity of 85%, and a moderate specificity of 47% for neonatal candidaemia.'161 Considering the high mortality for invasive fungal sepsis in neonates, it may be acceptable to use the candidaemia scoring model in empirical antifungal decision-making. The detection of (1,3)-ß-D glucan to diagnose invasive fungal sepsis in neonates has also been proposed, although very few studies have included neonates. If adult reference ranges are used, where >80 pg/ mL is considered positive, 60 - 80 pg/mL is considered equivocal, and <60 pg/mL is considered negative, the assay has a sensitivity of 70.7% and a specificity of 77.4%. These values should, therefore, be used as an adjunct to the clinical picture, haematological values, and the candidaemia score in the diagnosis of invasive fungal sepsis.[15] All patients with a positive fungal culture should have a full systemic evaluation (urine, blood, CSF, renal sonar, and fundoscopy) for invasive fungal sepsis.[16]
Sterile urine specimens should be collected for urinalysis and culture in neonates suspected to have LOS >6 days of life.[4,5,11,17] Although there are no clear criteria for the diagnosis of urinary tract infections (UTIs) in neonates,[18] many clinicians use the following guide: growth of any urinary pathogen (>1 000 CFU/mL) from a suprapubic specimen, or if a catheter specimen was taken, growth of >50 000 CFU/mL of a single uropathogen, or between 10 000 and 50 000 CFU/mL of a single uropathogen with evidence of pyuria.[11]
A lumbar puncture (LP) should be performed on all neonates with suspected sepsis, as nearly one-quarter of neonates with blood-culture-positive sepsis have concurrent meningitis,[4,5] and up to 38% of neonates with proven meningitis have a negative blood culture.[4,5] CSF indices indicating neonatal meningitis are controversial,[4] as there is considerable overlap between CSF values in neonates, with and without meningitis.[19,20] Adjusting the CSF white blood cell (WBC) count for the number of red blood cells in the CSF is unreliable, resulting in loss of sensitivity for only a slight gain in specificity.[4,5,7,20] Lab CSF values suggestive of neonatal meningitis include a WBC count >21 cellsμL,[7,20] with a sensitivity and specificity of ~80%.[7,20] The CSF protein varies inversely with gestational age,[4,5] with a value >1.5 g/L in a preterm neonate and >1.0 g/L in a term neonate being highly suggestive of bacterial meningitis.[20] Of all the variables, a low CSF glucose has the greatest specificity for meningitis.[4] The ratio of CSF to serum glucose is not useful in neonates, and a CSF glucose of <1.1 mmol/L in a preterm, and <1.7 mmol/L in a term neonate is suggestive of bacterial meningitis.[20] If the diagnosis of meningitis is questionable due to marginal WBC counts or other parameters, but the clinical picture is suspicious, the LP should be repeated within 24 - 48 hours.[7,20]
It remains controversial whether tracheal aspirates should be taken from neonates suspected to have ventilator associated pneumonia (VAP),[21] defined as the presence of a new pneumonia in a patient who has required assisted ventilation through an endotracheal tube in the previous 48 hours.[21, 22] Tracheal aspirates have a low sensitivity, low specificity, and poor positive predictive value in the diagnosis of VAP.[21] They may, however, identify the organisms colonising the airway, guiding the choice of antimicrobial therapy.[21] An organism may be considered significant if >106 CFU/mL are present.[23] A negative tracheal aspirate culture has a high negative predictive value for excluding a VAP.[21] The radiographic picture, clinical, and laboratory criteria may be used to make the diagnosis of VAP without a culture result.[21,22]
Osteomyelitis, although uncommon, is another consideration in a neonate suspected of sepsis, and may be accompanied by positive blood cultures in severe disease.[24]
Pathogenic organisms and contaminants
The most common organisms, accounting for ~70% of cases of EOS in the developed world, are Streptococcus agalactiae (Group B Streptococcus (GBS)) and Escherichia coli (E. coli).[1'3-5]The majority of LOS (70%) in the developed world is due to Gram-positive infections,[1,3,25] with CoNS,[6]Staphylococcus aureus, Enterococcus spp., and GBS being most common in VLBW infants.'31 Approximately 18 - 20% of LOS is due to Gram-negative infections (mostly Enterobacteriaceae spp.), and 12% due to fungal infections (Candida spp.).[3]
EOS in the developing world is most commonly caused by E. coli, Klebsiella spp. and S. aureus.[3]LOS is mainly caused by Grampositive organisms, such as CoNS,'61S. aureus, S. pneumoniae and S. pyogenes,[3] although the proportion of LOS caused by Gram-negative organisms is rising.[25] An SA study published in 2005 reported that Gram-negative organisms (E. coli) are responsible for the majority of EOS, and Gram-positive organisms (CoNS, Enterococcus faecalis, viridans streptococci) are the major contributors to LOS (57.9%).[8] This contrasts with two more recent SA studies, which reported that LOS and healthcare-associated infections are predominantly due to Gram-negative organisms, such as Acinetobacter spp., Klebsiella spp., Enterobacter spp., and E. coli (46.1 - 48.2%).[9,26] The most common Gram-positive organism was CoNS in one study,[9] and S. aureus and Enterococcus spp. in the other.[26] However, the result of the latter study may have been skewed, as all CoNS cultures were excluded and it was considered a contaminant.'[26]
Other pathogenic organisms include Listeria monocytogenes, Neisseria meningitides, N. gonorrhoeae, Haemophilus influenzae, Pseudomonas aeruginosa, members of the Bacteroides fragilis group, and Cryptococcus neoformans.[10]
Microorganisms that may be considered as contaminants in the majority of cases include Corynebacterium spp., Bacillus spp. other than Bacillus anthracis, Propionibacterium acnes, Micrococcus spp., and Clostridium perfringens.[10]
Identifying neonates at risk for sepsis
The need for empirical antimicrobial therapy is driven by the existence of risk factors for EOS, and clinical symptoms and signs for LOS.[1-3] Chorioamnionitis, maternal intrapartum pyrexia (temperature >38°C), prematurity (<37 weeks' gestation), low birth weight, assisted instrumental delivery, low Apgar scores, maternal colonisation with GBS or a previous neonate with a GBS infection, and prolonged rupture of membranes before delivery of >18 hours are all risk factors for EOS.[3-5] Prematurity, prolonged hospital admission, repeated invasive procedures, deep intravenous lines, intravenous lipids in total parenteral nutrition, prolonged antimicrobial administration, and mechanical ventilation are risk factors for LOS.[2,6]
Recommended empirical antimicrobial regimens
Knowledge of the most common pathogens and the antimicrobial susceptibility patterns is important to select the appropriate empirical antimicrobial(s).[3] Empirical antimicrobial therapy can be targeted when the culture result becomes available.[27]
In developed countries, all GBS isolates are sensitive to penicillin, ampicillin and vancomycin.[3] Ninety-six percent of E. coli isolates are sensitive to gentamycin or a cephalosporin, and 78% of E. coli isolates are resistant to ampicillin.[3] In combination, ~94% of EOS isolates (GBS, CoNS, non-pyogenic streptococci, and E. coli) are sensitive to a combination ofpenicillin plus gentamicin, and 100% of these organisms are sensitive to the combination of amoxicillin plus cefotaxime;[3] however, routine use of cefotaxime has been shown to rapidly increase bacterial resistance and prolonged use increases the risk for invasive fungal infections.[4,5] In SA, all GBS isolates are sensitive to ampicillin and 85.7% of E. coli isolates are sensitive to amikacin, with 100% sensitivity of both organisms to cefotaxime.™ Although empirical therapy for EOS should be individualised per hospital or region, a widely accepted empirical regimen is a combination of ampicillin plus an aminoglycoside.[3,4]
Empirical antimicrobial therapy for suspected LOS should, ideally, cover both Gram-positive and Gram-negative orga-nisms.[3,6] In developed countries, 95% of organisms causing LOS are sensitive to a combination of gentamicin with either amoxicillin or flucloxacillin,[6] or amoxicillin plus cefotaxime.[3] Only 79% of organisms are sensitive to cefotaxime alone.[3] In countries where invasive CoNS is increasing, vancomycin may be recommended as part of empirical therapy.[3] In developing countries, LOS due to gram-negative organisms, such as K. pneumoniae, E. coli, Pseudomonas spp., and Acinetobacter spp., is rising.[3] Approximately 70% of K. pneumoniae and E. coli are resistant to the combination of ampicillin plus gentamycin, and 50% are resistant to cefotaxime.[3]S. aureus is a common Gram-positive organism causing LOS, with methicillin resistance increasing.[3] In SA, ~40% of Gram-negative organisms are multidrug-resistant (75.6% Klebsiella spp., 86.5% Enterobacter spp., and 33.3% E. coli), including 14% of Acinetobacter spp. that are pan-drug resistant.[26] Gram-negative organisms are most resistant to ampicillin, gentamicin, and cefotaxime.[9] Of the Gram-positive organisms, 90 - 100% of S. aureus isolates and 55% of CoNS isolates are methicillin-resistant, and therefore require vancomycin.[8,9,26] Taking these antimicrobial resistance patterns into consideration, as well as the wide variety of organisms, there is insufficient evidence to recommend any antimicrobial regimen above another for the empirical management of suspected LOS.[3]
The combination of intravenous ampicillin plus an amino-glycoside should cover most urinary pathogens for the treatment of neonatal UTIs. Ampicillin may need to be substituted with vancomycin for the treatment of hospital-acquired UTIs, as the predominant organisms include CoNS, S. aureus and Enterococcus spp. Ideally, the urine culture should be repeated after 48 hours to document sterilisation.[11]
The recommended empirical antimicrobials for suspected early-onset meningitis are ampicillin plus an aminoglycoside[3,4] or ampicillin plus cefotaxime.[3,5] For late-onset meningitis, a combination of vancomycin plus a third-generation cephalosporin, with or without an aminoglycoside, is recommended.[3] Once the Gram stain, capsular antigen, or culture results are available, antimicrobials can be targeted.[28]
Empirical antifungal therapy must be determined by the institution's resistance pattern of fungal isolates, and can be targeted when the culture result is available.[14,16] The most common empirical antifungals used are amphotericin B and fluconazole, although fluconazole should not be used as empirical therapy if it had been administered as fungal prophylaxis.[16] Amphotericin B is the preferred antifungal for suspected fungal meningitis.[29,30]
Antimicrobial management of a VAP should be guided by local data on pathogens and sensitivity, and usually includes a broad-spectrum antimicrobial.[21,22]
Osteomyelitis requires a two-pronged management plan, including surgical drainage of pus and prompt initiation of antimicrobials targeted against the most common organisms. Gram-positive organisms, such as S. aureus, group A Streptococcus, GBS and alpha-haemolytic streptococci are responsible for most cases of osteomyelitis. Therefore, a combination of oxacillin plus an aminoglycoside is recommended as empirical therapy until culture results are available. If a unit has a high incidence of methicillin-resistant S. aureus (MRSA), oxacillin may be substituted with vancomycin.[24]
Antimicrobial duration
Stopping antimicrobials timeously is important as they disturb the neonate's faecal flora, disrupting the normal development of the nascent immune system.[7] Additionally, unnecessary antimicrobial administration is increasing antimicrobial resistance worldwide.
The appropriate duration of empirical antimicrobial therapy for culture-negative suspected EOS is debated, but standard practice is to stop antimicrobials if the culture remains negative for 48 - 72 hours and the patient has no clinical or haematological signs of infection.[3,5,6]
There are limited randomised controlled trials evaluating the outcome of shorter or longer antimicrobial courses for neonatal pneumonia and proven bacterial sepsis without meningitis or deep-seated infections. In a cohort of neonates of >32 weeks gestation and >1 500g, there was no difference in outcome or the need for readmission in the group receiving a short course (4 days) of antimicrobials for the management of pneumonia, provided the neonate was asymptomatic for the preceding 48 hours.[3] Antimicrobial durations of 7 - 14 days were compared in a similar group of neonates with blood-culture positive sepsis. As all of the neonates had a similar outcome, a duration of 7 days seems reasonable, provided the neonate is both clinically asymptomatic and has a CRP <10 mg/L by day 5 of treatment.[3] Longer antimicrobial courses of 10 - 14 days may still be appropriate for smaller or sicker neonates.[3]
Neonates with positive fungal blood cultures should receive an antifungal for a total duration of14 - 21 days after the first confirmed negative culture,[29] and fungal clearance should be confirmed by two negative blood cultures, 24 hours apart.[16] When amphotericin B is used, a cumulative dose of 25 - 30 mg/kg is recommended to prevent relapse.[29] The duration of empirical antifungals for culture-negative suspected fungal sepsis is unknown, but it is recommended to continue antifungals until the culture is reported as negative (incubated for at least 5 days).[12,16]
Definite data for the duration of treatment of neonatal UTI is lacking, but 7 - 14 days of antimicrobials are usually sufficient.[11,18] Renal ultrasound examination is recommended for all neonates diagnosed with the first UTI. [11,18]
Meningitis due to GBS should be treated for 14-21 days,[4] Listeria monocytogenes meningitis treated for >21 days and Gramnegative meningitis for >21 days.[3,4] Meningitis secondary to Grampositive organisms (other than GBS and L. monocytogenes) may be treated with 14 days of antimicrobials.[28] Currently, the adjunctive use of dexamethasone in neonatal bacterial meningitis cannot be recommended, owing to insufficient evidence.[3] Antifungals for confirmed fungal meningitis must be continued until the CSF parameters (protein, glucose, and WBC count) have returned to normal and the CSF culture is negative, which may take weeks to months.[30] Repeated LPs are not recommended, with the exception of neonates who do not respond adequately to 48 hours of treatment, and to document CSF sterilisation in neonates with Gram-negative[3] and fungal meningitis.[30]
The management of CSF culture-negative suspected meningitis should be guided by the blood culture result and CSF parameters.[20] If both the CSF and blood cultures taken prior to initiation of antimicrobials are negative, antimicrobial therapy may be discontinued after 48 - 72 hours.[28] Neonates with a positive blood culture, but negative CSF culture, and with raised CSF WBC should be treated for meningitis - 10 days of antimicrobials for Gram-positive bacteraemia and 14 days for Gram-negative bacteraemia.[28] If the LP was delayed and antimicrobials were administered, resulting in negative CSF and blood cultures, the duration of therapy should be guided by the CSF parameters.[28] If the CSF WBC count is raised, suggesting meningitis, antimicrobial therapy should be individualised (no standard duration), but if the CSF WBC is normal, antimicrobials can be discontinued after 48 - 72 hours.[28]
The combination of the clinical condition, biomarkers of infection, and radiographic picture can aid in determining when to discontinue antimicrobials for a VAP, although a duration of 7 - 10 days is usually sufficient.[22]
Osteomyelitis must be treated for a total duration of 4 - 6 weeks with intravenous antimicrobials; however, a longer duration of therapy is recommended if the causative organism is S. aureus (methicillin-sensitive or resistant).[24]
Antimicrobial stewardship
The core principles common to all antimicrobial stewardship (AMS) programmes are: (i) correctly identifying patients who need antimicrobial therapy; (ii) using local and regional antibiograms to guide prescribing; (in) avoiding antimicrobials with overlapping activity; (iv) administering the correct dose at the correct intervals; (v) regularly reviewing the culture results and adjusting antimicrobials appropriately; (vi) monitoring drug levels and adjusting the dose accordingly; and (vii) stopping antimicrobials promptly when guided by negative cultures.[13] The Department of Health Advisory Committee on Antimicrobial Resistance and Healthcare Associated Infection has developed the 'Start Smart - Then Focus' principles of AMS to guide clinicians on the appropriate use of antimicrobials (Table 2).[7]

Conclusion
Sepsis is a leading cause of death during the neonatal period, and clinicians must have a high index of suspicion, as neonates present with nonspecific clinical signs and symptoms. Early empirical antimicrobial treatment is associated with better outcomes in neonatal sepsis, but antimicrobials must be discontinued timeously to prevent the emergence of further antimicrobial resistance.
Acknowledgements. The authors would like to thank Prof. R J Green for his professional guidance and valuable support.
Author contributions. MC wrote the initial draft, critically reviewed the
manuscript, and participated in revising the manuscript. NTM critically reviewed the manuscript and participated in revising the manuscript. TWDW critically reviewed the manuscript and participated in revising the manuscript. All authors approved the final version to be published.
Funding. None.
Conflicts of interest. None.
References
1. Shah BA, Padbury JF. Neonatal sepsis: An old problem with new insights. Virulence 2014;5(1):1-9. https://doi.org/10.4161/viru.26906 [ Links ]
2. Dong Y, Speer CP. The role of Staphylococcus epidermidis in neonatal sepsis: Guarding angel or pathogenic devil? Int J Med Microbiol 2014;304:513-520. https://doi.org/10.1016/j.ijmm.2014.04.013 [ Links ]
3. Sivanandan S, Soraisham AS, Swarnam K. Review article: Choice and duration of antimicrobial therapy for neonatal sepsis and meningitis. Int J Pediatr 2011;2011:1-9. https://doi.org/10.1155/2011/712150 [ Links ]
4. Polin RA. Management of neonates with suspected or proven early-onset sepsis. Pediatr 2012;129(5):1006-1013. https://doi.org/10.1542/peds.2012-0541 [ Links ]
5. Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev 2014;27(1):21-47. http://doi.org/10.1128/CMR.00031-13 [ Links ]
6. Dong Y, Speer CP. Late-onset neonatal sepsis: Recent developments. Arch Dis Child Fetal Neonatal Ed 2014;100(3):F257-F263. https://doi.org/10.1136/archdischild-2014-306213 [ Links ]
7. Bedford Russel AR, Kumar R. Early onset neonatal sepsis: Diagnostic dilemmas and practical management. Arch Dis Child Fetal Neonatal Ed 2015;100(4):F350-F354. https://doi.org/10.1136/archdischild-2014-306193 [ Links ]
8. Motara F, Ballot DE, Perovic O. Epidemiology of neonatal sepsis at Johannesburg Hospital. South Afr J Epidemiol Infect 2005;20(3):90-93. [ Links ]
9. Lebea MM. Evaluation of culture-proven neonatal sepsis at a tertiary care hospital in South Africa (dissertation). University of the Witwatersrand, 2015. http://wiredspace.wits.ac.za/handle/10539/19970 (accessed 12 August 2016). [ Links ]
10. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev 2006;19(4):788-802. http://doi.org/10.1128/CMR.00062-05 [ Links ]
11. O'Donovan DJ. Urinary tract infections in neonates. UpToDate 2016. https://www.uptodate.com/contents/urinary-tract-infections-in-neonates (accessed 10 November 2016). [ Links ]
12. Doern GV. Blood cultures for the detection of bacteremia. UpToDate 2016. Available at: https://www.uptodate.com/contents/blood-cultures-for-the-detection-of-bacteremia (accessed 08 November 2016). [ Links ]
13. Patel SJ, Saiman L. Principles and strategies of antimicrobial stewardship in the neonatal intensive care unit. Semin Perinatol 2012;36(6):431-436. https://doi.org/10.1053/j.semperi.2012.06.005 [ Links ]
14. Benjamin DK, DeLong ER, Steinbach WJ, Cotton CM, Walsh TJ, Clark RH. Empirical therapy for neonatal candidemia in very low birth weight infants. Pediatrics 2003;112(3):543-547. https://doi.org/10.1542/peds.112.3.543 [ Links ]
15. Mackay CA, Ballot DE. Serum 1,3-ßD-glucan assay in the diagnosis of invasive fungal disease in neonates. Pediatric Reports 2011;3(2):45-48. https://doi.org/10.4081/pr.2011.e14 [ Links ]
16. Hsieh E, Smith B, Benjamin DK, et al. Neonatal fungal infections: When to treat? Early Hum Dev 2012;88(S2):S6-S10. https://doi.org/10.1016/s0378-3782(12)70004-x [ Links ]
17. Weisman LE, Pammi M. Clinical features and diagnosis of bacterial sepsis in the preterm infant (<34 weeks gestation). UpToDate 2016. http://www.uptodate.com/contents/clinical-features-and-diagnosis-of-bacterial-sepsis-in-the-preterm-infant-less-than34-weeks-gestation (accessed 08 August 2016). [ Links ]
18. Santoro JD, Carroll VG, Steele RW. Diagnosis and management of urinary tract infections in neonates and young infants. Clin Pediatr 2012;52(2):111-114. https://doi.org/10.1177/0009922812471713 [ Links ]
19. Majumdar A, Jana A, Jana A, Biswas S, Bhatacharyya S, Bannerjee S. Importance of normal CSF parameters in term versus preterm neonates. J Clin Neonatol 2013;2(4):166-168. http://doi.org/10.4103/2249-4847.123089 [ Links ]
20. Edwards MS. Bacterial meningitis in the neonate: Clinical features and diagnosis. UpToDate 2016. https://www.uptodate.com/contents/bacterial-meningitis-in-the-neonate-clinical-features-and-diagnosis. (accessed 7 November 2016). [ Links ]
21. Garland JS. Ventilator-associated pneumonia in neonates: An update. NeoReviews 2014;15(6):e225-e235. https://doi.org/10.1542/neo.15-6-e225 [ Links ]
22. Cernada M, Brugada M, Golombek S, Vento M. Ventilator-associated pneumonia in neonatal patients: An update. Neonatology 2014;105(2):98107. https://doi.org/10.1159/000355539 [ Links ]
23. Aelami MH, Lofti M, Zingg W. Ventilator-associated pneumonia in neonates, infants and children. Antimicrob Resist Infect Control 2014;3:30. https://doi.org/10.1186/2047-2994-3-30 [ Links ]
24. Fisher RG. Neonatal osteomyelitis. NeoReviews 2011;12(7):e374-e380. [ Links ]
25. Future directions in the evaluation and management of neonatal sepsis. NeoReviews 2012;13:e103-10. http://doi.org/10.1542/neo.12-7-e374 [ Links ]
26. Velaphi S, Wadula J, Madhi S. Pathogens isolated from blood stream of neonates diagnosed with healthcare associated infections: Antimicrobial susceptibility and case fatality rates. Presented at the 34th Conference on Priorities in Perinatal Care in South Africa, Drakensberg, South Africa, 17 - 20 March 2015. [ Links ]
27. Neonatal sepsis: An old issue needing new answers. Lancet 2015;15(5):503-505. http://dx.doi.org/10.1016/S1473-3099(14)71016-3 [ Links ]
28. Edwards MS. Bacterial meningitis in the neonate: Treatment and outcome. UpToDate 2016. https://www.uptodate.com/contents/bacterial-meningitis-in-the-neonate-treatment-and-outcome (accessed 8 November 2016). [ Links ]
29. Pammi M. Treatment of candida infection in neonates. UpToDate 2016. https://www.uptodate.com/contents/treatment-of-candida-infection-in-neonates (accessed 8 November 2016). [ Links ]
30. Kauffman CA. Candida infections of the central nervous system. UpToDate 2016. https://www.uptodate.com/contents/candida-infections-of-the-central-nervous-system (accessed 8 November 2016). [ Links ]
 Correspondence:
Correspondence:
M Coetzee
mel.coetzee@up.ac.za














