Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.11 n.2 Pretoria Jun. 2017
http://dx.doi.org/10.7196/SAJCH.2017.v11i2.1205
RESEARCH
Screening for retinitis in children with probable systemic cytomegalovirus infection at Tygerberg Hospital, Cape Town, South Africa
J F EngelbrechtI; N FreemanII; R M RautenbachIII
IMB ChB, Dip Ophth; Ophthalmology Department, Stellenbosch University, Tygerberg Hospital, Cape Town, South Africa
IIMB ChB , FC Ophth(SA), MMed Ophth Ophthalmology Department, Stellenbosch University, Tygerberg Hospital, Cape Town, South Africa
IIIMB ChB, FC Ophth(SA), MMed Ophth, MSc Med Ophthalmology Department, Stellenbosch University, Tygerberg Hospital, Cape Town, South Africa
ABSTRACT
BACKGROUND. The incidence of immunocompromised children with probable systemic cytomegalovirus (CMV) infection is increasing. Currently, there is no protocol for screening children for CMV retinitis in South Africa. Screening for CMV retinitis may prevent permanent visual impairment.
OBJECTIVES. To determine the prevalence of retinitis in children with probable systemic CMV infection. To assess the value of clinical and laboratory data in identifying risk factors for the development of CMV retinitis in children.
METHODS. A retrospective, cross-sectional study design was used. All children (< 2 years) with probable systemic CMV infection who underwent ophthalmic screening over a 5-year period, were included. Presumed CMV retinitis was diagnosed by dilated fundoscopy. All cases were evaluated to identify possible risk factors for the development of CMV retinitis.
RESULTS. A total of 164 children were screened. Presumed CMV retinitis was diagnosed in 4.9% of participants. Causes of immunosuppression were HIV infection (n=7) and chemotherapy (n=1). HIV infection showed a definite trend towards association with the development of CMV retinitis in our study population (p=0.064).
CONDUSION.The prevalence of CMV retinitis was 4.9% in our sample. Other than HIV, we were not able to identify additional risk factors for CMV retinitis. Our results show that CD4 levels are possibly not a reliable indicator to predict CMV retinitis.
Cytomegalovirus (CMV) infection may be due to vertical transmission (congenital CMV), or it may be horizontally acquired. Systemic CMV infection is more widespread in developing countries and in communities with a lower socioeconomic status, and it represents the most significant viral cause of birth defects in industrialised countries.[1] CMV infection is typically subclinical and therefore asymptomatic in healthy individuals; however, it can be life-threatening for the immunocompromised; such as HIV-positive patients, organ transplant recipients, and newborn babies.[2] Similar to other herpes viruses, CMV enters a latent state in which the virus is continually suppressed by cell-mediated immunity. CMV remains latent unless the patient suffers from a significant local or systemic immunodeficiency. Recurrent CMV infections are primarily associated with pneumonitis, colitis, encephalitis, and retinitis.[3] CMV retinitis is known to occur in immunocompromised adults, affecting up to 30% of HIV-positive adults and 5% of immunocompromised children.[4] CMV retinitis has been reported to be more likely in HIV-positive children with CD4 counts of <100 cells/pL, or CD4% < 10%.[4] While it is reported that CMV retinitis presents less frequently in children than in adults, children may not report visual loss or associated symptoms, making detection more difficult in the paediatric population.[5]
According to our literature review, the prevalence of retinitis in children with probable systemic CMV infection in South Africa (SA) is still unknown. Permanent visual impairment in children with CMV retinitis may be prevented by timely diagnosis and treatment. This study aimed to determine the prevalence of CMV retinitis, and to assess the value of clinical and laboratory data in identifying risk factors for the development of the disease in the study population.
Methods
Ethical considerations
The study was approved by the Health Research Ethics Committee of Stellenbosch University (ref. no. N13/01/012). Internationally accepted ethical standards and guidelines were respected and patient confidentiality was protected.
Study population and sampling
A retrospective, cross-sectional study design was used. All children S12 years admitted to Tygerberg Academic Hospital (TBH), with laboratory evidence of systemic CMV infection and who were referred for ophthalmological screening between 1 January 2009 and 31 December 2013, were included in the study. TBH is a tertiary-level referral hospital in Cape Town, SA. Ophthalmological screening consisted of a thorough examination of the central and peripheral retina by means of indirect ophthalmoscopy through dilated pupils. The screening was performed by a registrar and/or consultant in the Department of Ophthalmology. Presumed CMV retinitis was diagnosed on the characteristic clinical appearance on ophthalmoscopy. All cases with presumed CMV retinitis were reviewed by a consultant ophthalmologist. The study cohort was screened to determine the prevalence of presumed CMV retinitis.
All cases were evaluated to identify possible risk factors for the development of CMV retinitis. The immune status of all screened cases was evaluated and classified: nil immunodeficiency, prematurity, congenital immunodeficiency syndrome, perinatal vertical HIV exposure, HIV-positive organ transplantation, chemotherapy or other. Demographic, clinical and laboratory data of all children who underwent ophthalmological screening were collected and included the following: age, gender, T-lymphocyte subsets (including absolute CD4 and CD4/total lymphocyte count (CD4%)), site of systemic CMV infection, laboratory test type performed to detect CMV infection, and the nature of the specimen in which CMV was detected.
Laboratory analysis
Cases of probable systemic CMV infection were identified by CMV polymerase chain reaction (PCR), CMV pp65 antigenaemia test, CMV viral culture, and CMV serology. If the qualitative PCR was positive, quantitative PCR was performed. Probable systemic CMV infection had an accompanying quantitative PCR that was higher than detectable limits. HIV was diagnosed by means of HIV PCR in children <18 months of age, and by PCR or viral antibody tests for children >18 months. CD subsets were analysed to determine the absolute CD4 and CD4%. All white cells were counted and marked with CD3, CD4, and CD8 antibodies to determine the absolute CD count. CD4% was calculated by analysing the cells that exclusively contained the marker as a percentage of the total cells. Laboratory tests were performed by the National Health Laboratory Service.
Data management and statistical analysis
IBM SPSS version 22 (IBM Corp., USA) was used to analyse the data. The data were summarised using mean and standard deviation in the case of normally distributed continuous data, and frequency tables and percentages in the case of categorical data. The prevalence of CMV retinitis in children with probable systemic CMV infection was calculated. Associations between the risk factors identified and the development of CMV retinitis were assessed using Fisher's exact 2-sided tests for categorical variables, and non-parametric Mann-Whitney tests were used to compare non-normally distributed continuous variables between the groups.
Results
Demographic and clinical data profile of the sample
A total of 164 cases with probable systemic CMV infection were referred for ophthalmological examination to assess the presence or absence of CMV retinitis. The median age was 3.1 months (range 1 day to 131.6 months), and the ratio of male to female was 1.1:1. The most common sites of presumed CMV infection were the respiratory system (n=122), hepatobiliary system (n=17), cardiovascular system (n=8), central nervous system (n=6), and gastrointestinal tract (n=3) (Table 1).
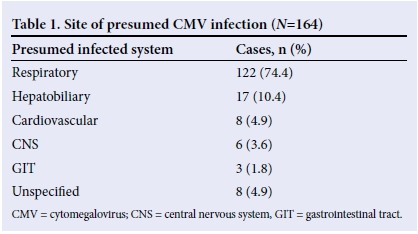
Underlying immunodeficiency
We identified 91 cases that were immunocompromised. Causes for immunosuppression were HIV infection (n=84), other immunodeficiency (n=6), and chemotherapy for leukaemia (n=1) (Fig. 1).
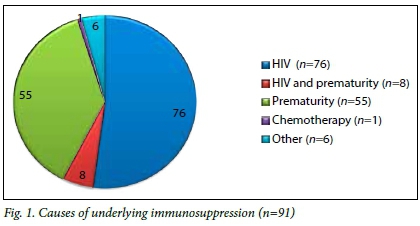
HIV infection was diagnosed in 51.2% (n=84) of the screened cases. In our study population there was a definite trend towards association between HIV infection and the development of CMV retinitis (p=0.064). However, due to small numbers, this association could not be confirmed. Highly active antiretroviral therapy (HAART) was initiated 78 (93%) participants of the HIV-positive or HIV-exposed cases at the time of screening. A total of 55 (33.5%) premature infants were screened.
CMV retinitis group
CMV retinitis was diagnosed in 4.9% (n=8) of the cases screened. Age ranged from 3 months to 11 years and the male to female ratio was 1:4. The causes of immunosuppression in this group were HIV infection (n=7) and chemotherapy for leukaemia (n=1). All of the HIV-infected patients were on HAART. Two of the HIV-positive cases were also premature, but due to small numbers the significance of prematurity as a risk factor for CMV retinitis could not be proven.
Laboratory data
Laboratory evidence of CMV infection was confirmed by the following tests: CMV PCR (n=136), CMVpp65 antigenaemia test (n=15), CMV viral culture (n=9), and CMV serology (n=4). All qualitative CMV PCR tests had an accompanying quantitative PCR higher than detectable limits. The HIV-positive group had a median absolute CD4 cell count of 598 cells^L between HIV-positive cases with and without CMV retinitis (p=0.032). The median CD4 cell count in those with CMV retinitis was 334 (range 21-767), while in those without retinitis it was 622 cells/ μL. CD4 as a percentage of the total lymphocyte count (CD4%) ranged between 2.1 and 64.3%, with a median of 16.6%. There was no significant difference between those with and without CMV retinitis in terms of CD4% (p=0.668).
Discussion
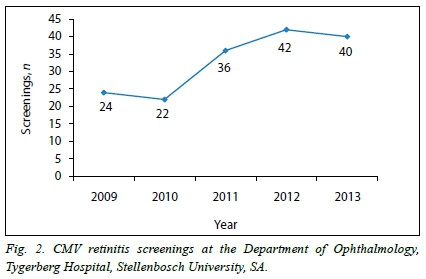
There was an exponential increase in the number of paediatric cases with probable systemic CMV infection screened for retinitis at the Department of Ophthalmology, TBH, SA over a 5-year period (Fig. 2), with a resultant increased load on our already overburdened ophthalmology service.
In 2006, the estimated number of SA children infected with HIV was 293 000.[6] Due to the HIV pandemic, paediatric CMV infection is becoming more prevalent and CMV infection has been identified in up to 51% of HIV-positive children admitted to SA paediatric intensive care units.[7] Patients on immunosuppressive therapy or with immunodeficiency syndromes are predisposed to CMV infections.[5] The distinctive features of active CMV retinitis are: 'a fulminant picture of retinal vasculitis and vascular sheathing with areas of yellow-white, full thickness, retinal necrosis producing retinal oedema associated with haemorrhage and hard exudates'.[8] The indolent variant is described as having less oedema and no haemorrhage or vascular sheathing.[8] In children, CMV retinitis is most often bilateral and can lead to a destructive retinitis of the posterior pole, resulting in permanent visual impairment.[8] Recent developments have led to rapid laboratory detection techniques for systemic CMV and treatment regimens for CMV infection have been improved and are more readily available.[9] Current treatment regimens include intravenous ganciclovir (6 mg/kg, 12 hourly) as the drug of choice, and oral valganciclovir and intravenous foscarnet as alternatives.[4] Although CMV-specific antiviral treatment may cause regression of retinitis, visual acuity may not improve due to macular or optic nerve involvement. Prolonged CMV antiviral treatment is needed to reduce recurrences of retinitis in children that remain immunocompromised.[10]
Several international studies have reported the incidence of CMV retinitis in HIV-positive cases: Rwanda 1.8%;[11] USA 2.3%[12]; and Canada 5%.[10] To our knowledge, this is the first study in SA to document the prevalence of presumed CMV retinitis in children with presumed systemic CMV infection. The gold standard for detecting congenital CMV in newborn children is viral isolation in the urine or saliva within the first 3 weeks of life, but serum PCR is reported to be equally sensitive and specific.[9] All the cases in our study population were diagnosed after the age of 3 weeks. We were therefore not able to distinguish congenital from acquired CMV infection.
Gender, prematurity, and site of systemic CMV infection were not significant risk factors for the development of retinitis in our paediatric sample. Screening for CMV retinitis in HIV-positive children with probable systemic CMV is imperative in South Africa. In our sample, 7 of the 8 CMV retinitis cases identified were HIV-positive. Only one child in the HIV-negative group developed retinitis; this patient was on chemotherapy for the treatment of leukaemia. CD4 count and CD4% may prove to be valuable in identifying cases at high risk of developing CMV retinitis;[4] however, we could not confirm this association in our study. We would therefore recommend that caution be taken when using CD4 and/or CD4% as criteria for retinitis screening as some cases might remain undiagnosed. International guidelines reported that CD4 cell level is less predictive of risk for CMV disease in young infants with HIV, and CMV infection can occur in HIV-infected children with higher CD4 counts.[4] With appropriate protocols in place, children could be screened and diagnosed early, managed effectively, and possibly have more favourable visual outcomes.
Conclusion
The prevalence of probable CMV retinitis in our study population was 4.9%. Other than HIV, we were not able to identify additional risk factors for CMV retinitis. Our results show that CD4 levels are possibly not a reliable guide to predict CMV retinitis. We recommend a multi-centre study to establish a robust screening protocol that may lessen the impact of the ever-increasing numbers of children at risk of CMV retinitis on ophthalmic services.
Acknowledgements. The authors would like to thank Dr J Maritz (Department of Virology, Stellenbosch University) for his contributions.
Author contributions. None.
Funding. None.
Conflict of interest. None.
References
1. Caruso C, Buffa S, Candore G, et al. Mechanisms of immunosenescence. Immun Ageing 2009;6(1):10. https://doi.org/10.1186%2F1742-4933-6-10 [ Links ]
2. Kenneth JR, Ray CG. Sherris Medical Microbiology. 4th ed. New York: McGraw-Hill, 2003;439-451. [ Links ]
3. Gallant JE, Moore RD, Richman DD, et al. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. J Infect Dis 1992;166(6):1223-1227. https://doi.Org/10.1093%2Finfdis%2F166.6.1223 [ Links ]
4. Mofenson LM, Brady MT, Danner SP, et al. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV- infected children: Recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2009;58(RR-11):1-166. https://doi.org/10.1086%2F427295 [ Links ]
5. Du LT, Coats DK, Kline MW, et al. Incidence of presumed cytomegalovirus retinitis in HIV-infected pediatric patients. J Am Assoc Pediatr Opthalmol 1999;3(4):245-249. https://doi.org/10.1016%2Fs1091-8531%2899%2970010-8 [ Links ]
6. Meyers T, Moultrie H, Naidoo K, Cotton M, Eley B, Sherman G. Challenges to pediatric HIV care and treatment in South Africa. J Infect Dis 2007;196(s3):S474-S481. https://doi.org/10.1086%2F521116 [ Links ]
7. Rabie H, de Boer A, van den Bos S, Cotton MF, Kling S, Goussard P. Children with human immunodeficiency virus infection admitted to a paediatric intensive care unit in South Africa. J Trop Pediatr 2007;53(4):270-273. https://doi.org/10.1093%2Ftropej%2Ffmm036 [ Links ]
8. Wren SME, Fielder AR, Bethell D, et al. Cytomegalovirus retinitis in infancy Eye 2004;18(4):389-392. https://doi.org/10.1038%2Fsj.eye.6700696 [ Links ]
9. Nassetta L, Kimberlin D, Whitley R. Treatment of congenital cytomegalovirus infection: Implications for future therapeutic strategies. J Antimicrob Chemother 2009;63(5):862-867. https://doi.org/10.1093%2Fjac%2Fdkp083 [ Links ]
10. Baumal CR, Levin AV, Read SE. Cytomegalovirus retinitis in immunosuppressed children. Am J Ophthalmol 1999;127(5):550-558. https://doi.org/10.1016%2Fs0002-9394%2899%2900031-8 [ Links ]
11. Kestelyn P, Lepage P, Karita E, van de Perre P. Ocular manifestations of infection with the human immunodeficiency virus in an African pediatric population. Ocul Immunol Inflamm 2000;8(4):263-273. https://doi.org/10.1076%2Focii.8.4.263.6455 [ Links ]
12. Chandwani S, Kaul A, Bebenroth D, et al. Cytomegalovirus infection in human immunodeficiency virus type 1-infected children. Pediatr Infect Dis J 1996;15(4):310-314. https://doi.org/10.1097%2F00006454-199604000-00006 [ Links ]
 Correspondence:
Correspondence:
J F Engelbrecht
edrich.engelbrecht@gmail.com














