Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Child Health
versión On-line ISSN 1999-7671
versión impresa ISSN 1994-3032
S. Afr. j. child health vol.11 no.2 Pretoria jun. 2017
http://dx.doi.org/10.7196/SAJCH.2017.v11i2.1167
RESEARCH
Prevalence of and risk factors for cranial ultrasound abnormalities in very-low-birth-weight infants at Charlotte Maxeke Johannesburg Academic Hospital
A GhoorI; G ScherII; D Ε BallotIII
IMB ChB Department of Paediatrics and Child Health, University of the Witwatersrand and Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
IIMB BCh, FC Paed (SA), MMed (Paed) Department of Paediatrics and Child Health, University of the Witwatersrand and Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
IIIMB BCh, FCPaed (SA), PhD Department of Paediatrics and Child Health, University of the Witwatersrand and Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
ABSTRACT
BACKGROUND. Periventricular-intraventricular haemorrhage (IVH) and cystic periventricular leukomalacia (cPVL) contribute to neonatal mortality and morbidity. Low birth weight and gestational age are among the risk factors for IVH and cPVL.
OBJECTIVES. To assess how many very low birth weight (VLBW) infants had cranial ultrasound screening at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) and to determine the prevalence of cranial ultrasound abnormalities. To compare the characteristics and risk factors of those VLBW infants with cranial ultrasound abnormalities to those with normal cranial ultrasound findings.
METHODS. This was a retrospective case-controlled study of infants <1 500 g admitted to CMJAH from 1 January 2013 to 31 December 2015. Cases were identified as infants with IVH or cPVL. Controls were matched 1:2 based on birth weight and gender.
RESULTS. Only 55% (856/1 562) of VLBW infants had undergone cranial ultrasound screening. The final sample included 803 VLBW infants. IVH was identified in 26.7% of cases (n=215; 95% confidence interval (CI) 23.8 - 29.9) and 0.9% had cPVL (n=8; 95% CI 0.5 - 1.9). A total of 197 cases were identified and matched with 394 controls. Antenatal care attendance was lower in the cases (71% v. 79%; p=0.039). Sepsis, ventilation, metabolic acidosis and patent ductus arteriosus were all significantly higher in the cases. The use of antenatal steroids was significantly higher in the grades I - II IVH/no-IVH group v. grades III - IV IVH group (44% v. 25%;p=0.017).
CONCLUSION. The prevalence of IVH in our setting was consistent with that of developed countries. Improving antenatal care, infection control, and adequate early resuscitation could decrease the incidence of IVH and cPVL. All VLBW infants should undergo cranial ultrasound screening.
Periventricular-intraventricular haemorrhage (IVH) and white matter injury (WMI), particularly cystic periventricular leukomalacia (cPVL), are major contributors to mortality and long-term morbidity in preterm infants.'[1,2] The immature blood-brain barrier combined with fluctuation in cerebral blood flow, or platelet and coagulation disorders, form the basis for IVH pathogenesis. Risk factors for IVH include low birth weight (LBW), gestational age (GA), prematurity, lack of antenatal steroids, asphyxia, prolonged labour, respiratory distress syndrome, recurrent tracheal suctioning, hypoxia, hypercarbia, acidosis, patent ductus arteriosus (PDA), rapid infusion of sodium bicarbonate and inter-hospital transfer.[3] Preterm infants with cPVL, without IVH, appear to have a different risk pattern, although the pathogenesis does involve both hypoxic-ischaemic and haemorrhagic processes. It also appears that intrauterine inflammation and cytokine release result in oxidative stress to white matter.[4, 5]
IVH can be graded according to severity, from grades I to IV, with the most commonly used classification being that of Papile et al.[6] The incidence of IVH in developed countries is about 20 - 25% in very low birth weight (VLBW) infants. More than 50% of those with severe IVH develop intellectual disability or cerebral palsy.[7] Research in southern Africa showed a higher incidence of between 20% and 50% of IVH in VLBW infants, although the prevalence of severe IVH is similar to that of developed countries.[8, 10]
Most neonatal units have a screening protocol in place to detect IVH and cPVL. The American Academy of Neurology suggests that cranial ultrasound screening should be performed at two time points for all infants with a GA <30 weeks: between weeks 1 and 2 of life, and at 36 - 40 weeks postmenstrual age.[7] More recent studies have shown that magnetic resonance imaging (MRI) performed at the term equivalent age could be helpful in identifying white matter abnormalities that could cause neurocognitive delay as a long-term outcome.[11] However, cranial ultrasound is still the first tool for assessing neurological injury in preterm infants, as it allows for bedside investigation and sequential evaluation of evolving lesions.
As neonatal and obstetric care improves, there is an increase in the survival rate of preterm infants, and an associated newer burden of disease in the form of neurodevelopmental impairment.[2, 12] In order to improve the screening and management of IVH at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH), we needed to investigate the prevalence of IVH and cPVL, and identify the specific risk factors important in our population. LBW and GA are risk factors for IVH, therefore we chose to conduct a study that controlled for these possible confounders.[13]
Our aim was to assess how many VLBW infants had cranial ultrasound screening and to determine the prevalence of cranial ultrasound abnormalities in our VLBW population. We also wanted to compare the characteristics, risk factors, and short-term outcomes of VLBW infants with cranial ultrasound abnormalities with those of VLBW infants, with normal cranial ultrasound results.
Methods
Data from the CMJAH neonatal database were used in this retrospective, case-controlled study. The study population included all VLBW infants admitted to the neonatal unit, including the intensive care unit, from 1 January 2013 to 31 December 2015. CMJAH is a tertiary hospital located in Johannesburg, South Africa (SA). It acts as a referral centre for surrounding clinics and district hospitals. The study population included both inborn and outborn infants. Infants born with a birth weight <1 500 g qualified as VLBW. All VLBW infants who had the following ultrasound abnormalities were identified as cases: IVH, post-haemorrhagic hydrocephalus, or cPVL. Cases were matched with control infants based on birth weight category and gender on a 1:2 basis.
The two most suitable controls born closest to the study patient were selected. Grading of IVH was based on the classification described by Papile et al:l6]grade I included bleeds restricted to the germinal matrix; grade II bleeds were those extending into the ventricles and filling -50% of the ventricle; grade III bleeds caused ventricular dilatation; and grade IV included parenchymal haemorrhage. Grade III and IV bleeds constitute severe IVH. There were four birth weight categories: <750 g; 751 - 1 000 g; 1 001 - 1 250 g; and 1 251 - 1 500 g. Infants with major congenital abnormalities of any organ system were excluded. Infants with cranial ultrasound revealing abnormalities such as absent corpus callosum, schizencephaly or findings with questionable significance, such as periventricular echodensities, were also excluded.
Data collection
Data were collected from the neonatal database at CMJAH, which is stored using REDCap (Research Electronic Data Capture).[14] It is a secure web-based application that is linked to the Vermont Oxford Network (VON).[15] The VON includes ~1 000 centres around the world that submit data about high-risk newborn infants. It is a nonprofit voluntary collaboration of health professionals aiming to improve neonatal care. Its members include both public and private units from North America, South America, Europe, the Middle East, Asia and southern Africa, to name a few. Sixty-four units in SA are members of the VON.
The following maternal variables were extracted from the database for each infant: antenatal care attendance, antenatal steroids, magnesium sulphate administration, chorioamnionitis, HIV, attempted termination of pregnancy, hypertension, diabetes, and mode of delivery. Newborn variables included the place of birth (inborn, midwife obstetric unit (MOU), another hospital, or home), GA, birth weight, head circumference, gender, multiple gestation, Apgar at 5 min, birth resuscitation, temperature on admission, and metabolic acidosis. Birth resuscitation included oxygen, bag mask ventilation, intubation, cardiopulmonary resuscitation and/or intravenous adrenaline. Ultrasound findings were also recorded, particularly grade of IVH and presence of cPVL. The neonatal course was documented and included respiratory diagnosis, respiratory support, days on continuous positive airway pressure (CPAP), days mechanically ventilated, surfactant therapy, supplementary oxygen on day 28, indomethacin or Ibuprofen exposure, and bacterial sepsis (early or late). Early sepsis constituted a positive blood culture within 72 hours of birth and late sepsis was a positive blood culture >72 hours after birth. Other neonatal diagnoses noted were pneumothorax, PDA, necrotising enterocolitis, neonatal jaundice, blood transfusion, HIV testing and major birth defects. Short-term outcome, namely survival to discharge, was also recorded. Infants who were well enough to be transferred to other hospitals were considered survivors.
Statistical methods
Statistical analysis was performed using SPSS Statistics version 23 (IBM Corp., USA). Data were described using standard statistical methods. Continuous data with a normal distribution were described using means and standard deviations (SDs), while skewed data were described using median and range values. Categorical variables were described using frequencies and percentages. Univariate analysis was performed to describe differences between the case and control groups using the Pearson χ2or Fisher's exact test for categorical variables. Continuous variables were compared using independent f-tests or non-parametric tests (Mann-Whitney) as appropriate, depending on data distribution. Basic demographic data of the group who had not undergone cranial ultrasound were compared with the final study sample that had undergone ultrasound to establish if the selected sample was random. Sub-analysis also included comparison between those with mild/no IVH with those with severe IVH and cPVL, respectively. A p-value i 0.05 was considered significant. The variables on univariate analysis with p<0.1 were analysed further in a stepwise logistic regression model to determine risk factors for IVH and cPVL. Only valid cases were reported for all variables in the analysis, i.e. missing data were excluded.
Ethics
The Human Research Ethics Committee at the University of the Witwatersrand, Johannesburg, approved the study (ref. no. M151195).
Results
There were 1 562 VLBW infants born between 1 January 2013 and 31 December 2015 at CMJAH. Cranial ultrasounds were performed on 856 (55%) infants. Table 1 shows the prevalence of cranial ultrasound abnormalities at CMJAH. Of the 856 patients with cranial ultrasounds, 13 were excluded from the study due to major birth defects. The remaining infants (N=843) who underwent cranial ultrasound were then divided into normal (N=580) and abnormal (N=263) cranial ultrasound groups. Forty infants in the abnormal group (15%) were excluded due to sonar findings that did not meet inclusion criteria. A further 26 infants with abnormal cranial ultrasounds had no matching controls with the correct weight category or gender and were excluded. There were 186/580 (32%) in the normal group who could not be matched with an abnormal' infant. There were therefore 197 cases and 394 controls selected from the infants who had undergone cranial ultrasound (Fig. 1).


Basic demographic data showed that 47% (729/1 562) of infants admitted were males, median birth weight was 1 150 g and mean gestational age was 29 weeks. The mortality of the overall group was 27% (415/1 562). There were fewer males in the group who had not undergone ultrasound (417/702; 45%) compared with the case/control group (303/591, 51%;p=0.029). The median weight was higher in the group with no ultrasound (1 180 g v. 1 090 g in the case/control group; p=0.05). The mean GA of 29 weeks was the same for both groups. Mortality in the group who had not undergone ultrasound was higher (39% v. 17%). This result had a significant p-value of 0.026. The higher mortality in this group could be accounted for by the large number of infants who were <750 g and who had not undergone ultrasound (13% v. 3%). When comparing cases and controls, we found that the gender distribution was the same, with males constituting 49% in both groups. The mean birth weight was 1 097 g for cases and 1 065 g for controls. The mean gestational age was 29 weeks for both groups. The above parameters had no statistical significance, which confirmed that cases and controls were matched. The data in Table 1 include all those VLBW infants who had a cranial ultrasound and were included in the study (N=803) to calculate the prevalence over the specified time period. The data in Tables 2-5 include only those VLBW infants selected as cases (n=197) or controls (n=384).
The univariate comparison between case and control groups is shown in Table 2. A higher proportion of cases were outborn. Sepsis, metabolic acidosis, ventilation and PDA were all significantly higher in the cases, and antenatal care and Apgar at 5 minutes were lower. However, there was no significant difference between the two groups in the use of antenatal steroids.
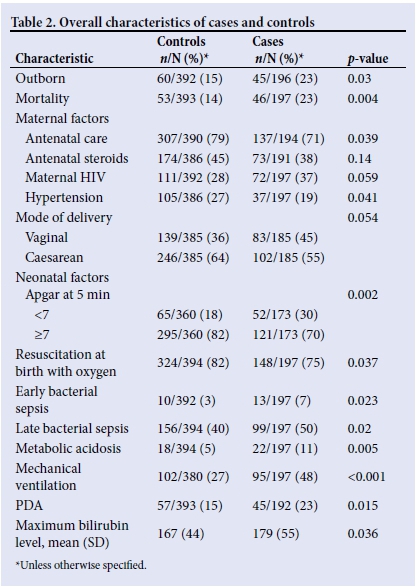
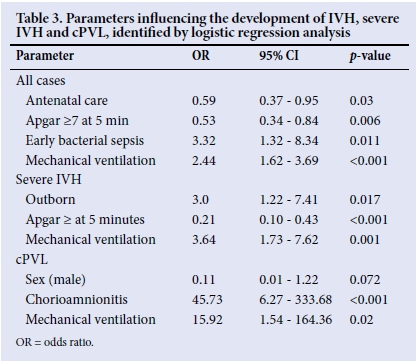
The logistic regression analysis included location of birth, antenatal care, maternal hypertension, maternal HIV, mode of delivery, Apgar at 5 minutes, initial resuscitation with oxygen, early and late bacterial sepsis, PDA and ventilation (Table 3). The analysis showed that antenatal care attendance (odds ratio (OR) 0.59; 95% CI 0.37 - 0.95) and Apgar >7 at 5 minutes (OR 0.53; 95% CI 0.34 - 0.84) were associated with a lower risk of abnormal cranial ultrasound. Ventilation carried a higher risk of cranial ultrasound abnormality (OR 2.44; 95% CI 1.62 - 3.69), and early bacterial sepsis followed this trend (OR 3.32; 95% CI 1.3 - 8.34). The rest of the variables were not significantly different between the groups.
Sub-analyses
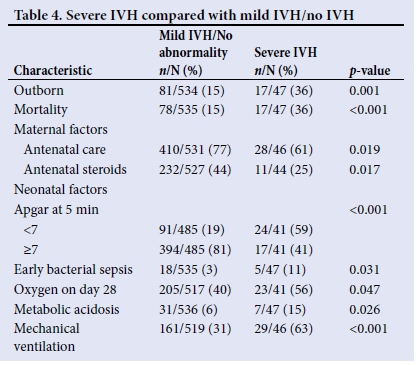
Severe IVH within the selected cases was compared with mild/no IVH within the case and control groups. Significant results are shown in Table 4. The use of antenatal steroids was higher in the mild/no IVH group (44%) compared with the severe IVH cases (25%; p=0.017).
Logistic regression analysis revealed that both outborn and ventilated VLBW infants had a higher risk of severe IVH. An Apgar score of >7 had indicated a lower risk of severe IVH (Table 3). The sample of infants with cPVL was compared with those with mild/no IVH (Table 5). The significant factors associated with cPVL on univariate analysis were sex (female), chorioamnionitis, blood transfusion, late bacterial sepsis, as well as ventilation. Logistic regression analysis (Table 3) revealed that chorioamnionitis (OR 45.73; 95% CI 6.27 - 333.68) and ventilation (OR 15.92; 95% CI 1.54 - 164.36) hold high risks of cPVL.
Discussion
The main objectives of this study were to analyse the prevalence of cranial ultrasound abnormalities and associated risk factors. According to the screening protocol at CMJAH, every VLBW should have an ultrasound within the first 7 days of life, at 10 - 14 days of life, and before discharge.1161 Only 55% of VLBW infants admitted during the study period had a cranial ultrasound, much lower than the prevalence in the VON database, where 90% of VLBW infants had cranial sonars. [15] Reasons for the low coverage rate in our unit included staff shortages at times, but more importantly, the lack of appropriate equipment to perform ultrasound. The unit only has one sonar machine that is often not functional.
The patients who did not undergo cranial ultrasound had a higher mortality rate than those who had undergone an ultrasound. This is partly explained by the fact that 13% (n=92) of infants in that group were < 750 g. These infants make up a high-risk group related to their extreme prematurity and are likely to have demised before an ultrasound could be performed.
VLBW infants who were admitted with higher birth weights were often well enough to be transferred to the kangaroo mother care ward and were discharged quickly, without undergoing cranial ultrasound screening.
The prevalence of IVH (26%) appears to be consistent with that of studies conducted in developed countries and was similar to that reported in the VON over the same time period.'71 With improvement in perinatal care, the prevalence appears to have decreased when compared with a study done at our sister unit at Chris Hani Baragwanath Hospital in Soweto 20 years ago, which showed a prevalence of 52% in VLBW infants.[8] The prevalence of severe IVH in the present study (7%) is less than that reported in the VON, and studies from both developed and developing countries.[79]
This study confirms what is already known: infants with IVH have higher mortality, and lack of antenatal care, birth asphyxia, and sepsis appear to be associated with the development of IVH. Mechanical ventilation and PDA also had an increased association with IVH and this can be explained by fluctuations in cerebral blood flow.[3] The prevalence of IVH in inborn patients was less compared with those who were outborn, and there are many factors that could explain the higher risk, including delay in transfer, inadequate monitoring, and lack of appropriate management at referring centres. Contrary to other studies, which showed a decrease in IVH with antenatal steroids, our study did not show a significant difference between the use of antenatal steroids in the case and control groups.[3] This may be due to the fact that the administration of antenatal steroids in our setting was lower than in other settings. Only 43% of mothers of VLBW infants had received steroids compared with 83.5% of those in the VON during the same period, which may have been due to late presentation of unbooked mothers.[17] However, the sub-analysis showed that those with severe IVH had less exposure to antenatal steroids than the mild/ no IVH group.
Maternal hypertension was also associated with a lower incidence of overall IVH and this was consistent with other studies.[13] In terms of the logistic regression analysis for all IVH cases, the major protective factors were antenatal care and Apgar >7 at 5 minutes. Sepsis and mechanical ventilation showed a ~3-fold increase in IVH and both of those factors can be modified by preventive measures. Severe IVH had similar association on logistic regression analysis to overall IVH, but infants that were outborn were shown to have a 3-fold higher risk of severe IVH, which could be prevented by transport of mothers in premature labour to centres with appropriate neonatal services, before delivery.
In this study, the prevalence of cPVL was only 1%, although mortality was highest in this group, which was consistent with previous studies.[2] As explained previously, MRI is a better tool to assess WMI, but in our setting this was not feasible owing to a lack of adequate MRI facilities to service the neonatal unit. Studies seeking to describe a difference between cPVL and IVH have shown that the two are not mutually exclusive and that there is a possible causal relationship between the two, although cPVL can be seen as a single pathology in some cases.[4,5]
Our sub-analysis showed that the female gender, chorioamnionitis, and blood transfusion had significant associations with cPVL, as well as sepsis and mechanical ventilation, which were both additional risk factors for IVH. However, on multivariate logistic regression analysis, the gender association was shown to have an insignificant 95% CI and only chorioamnionitis and mechanical ventilation appeared significant, although CIs were wide owing to the small sample size.
Study limitations
The retrospective study design was a limitation. The disadvantages included information bias and difficulty in establishing the temporal relationship between certain variables. Incomplete and/or missing records affected the dataset. Another limitation was the lack of resources, including both staff and equipment, which resulted in deviations from the ultrasound screening protocol and 45% of VLBW infants not being screened. WMI may be largely underestimated by cranial ultrasound alone. In our setting, the access to MRI was inadequate.
Conclusion
This retrospective, matched case-control study showed that the prevalence of IVH was consistent with that of developed countries and it was encouraging to note that severe IVH was lower in our unit. It confirmed that the risk factors for IVH and cPVL at CMJAH are in keeping with those found globally. The increased mortality and morbidity in infants with IVH would suggest that we should modify the risk factors found to be significant in our population. Access and awareness of early antenatal care is an important factor in preventing poor perinatal outcome. Neonatal resuscitation training in clinics and hospitals should be given priority, as well as correct protocols for transfer of infants, and possible early transfer of mothers in preterm labour to appropriate centres. Infection control and methods to decrease the need for mechanical ventilation are important interventions that could decrease the incidence of IVH. In terms of ultrasound screening, early detection of lesions gives infants a chance to receive early intervention services. Therefore, efforts should be made to ensure that all VLBW infants undergo ultrasound screening. Furthermore, early identification of patients with poor prognostic factors could assist with decisionmaking regarding the maximum level of care offered and the equitable allocation of resources.
Acknowledgements. The authors thank all the staff involved in data collection in the neonatal unit at CMJAH.
Author contributions. AG, GS and DEB designed the study and developed the methodology. AG collected the data, performed the analyses and prepared the manuscript. The process was supervised by DEB and GS. The final manuscript was reviewed and edited by DEB and GS.
Funding. This study was funded by the authors.
Conflicts of interest. None.
References
1. Brouwer AJ, Groenendaal F, Benders MJNL, de Vries LS. Early and late complications of germinal matrix-intraventricular haemorrhage in the preterm infant: what is new? Neonatology 2014;106(4):296-303. http://dx.doi.org/10.1159/000365127 [ Links ]
2. Whyte HE, Blaser S. Limitations of routine neuroimaging in predicting outcomes of preterm infants. Neuroradiology 2013;55(Suppl 2):3-11. http://dx.doi.org/10.1007/s00234-013-1238-6 [ Links ]
3. Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol 2014;41(l):47-67. http://dx.doi.org/10.1016/jxlp.2013.09.007 [ Links ]
4. Larroque B, Marret S, Ancel PY, et al. White matter damage and intraventricular hemorrhage in very preterm infants: The EPIPAGE study. J Pediatr 2003;143(4):477-483. http://dx.doi.org/10.1067/S0022-3476(03)00417-7 [ Links ]
5. Küsters CDJ, Chen ML, Follett PL, Dammann O. 'Intraventricular' hemorrhage and cystic periventricular leukomalacia in preterm infants: How are they related? J Child Neurol 2009;24(9):1158-1170. http://dx.doi.org/10.1177/0883073809338064 [ Links ]
6. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1 500 g. J Pediatr 1978;92(4):529-534. [ Links ]
7. McCrea HJ, Ment LR. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol 2008;35(4):777-792. http://dx.doi.Org/10.1016/j.clp.2008.07.014 [ Links ]
8. Sandler DL, Cooper PA, Bolton KD, Bental RY, Simchowitz ID. Periventricular- intraventricular haemorrhage in low-birth-weight infants at Baragwanath Hospital. S Afr Med J 1994;84(1):26-29. [ Links ]
9. Mulindwa MJ, Sinyangwe S, Chomba E. The prevalence of intraventricular haemorrhage and associated risk factors in preterm neonates in the neonatal intensive care unit at the University Teaching Hospital, Lusaka, Zambia. Med J Zambia 2012;39( 1):1-6. [ Links ]
10. Adhikhari M. Cranial imaging in very low birth weight babies: A review. Obstet Gynaecol Forum 2008;18(3):87-90. [ Links ]
11. Benders MJ, Kersbergen KJ, de Vries LS. Neuroimaging of white matter injury intraventricular and cerebellar hemorrhage. Clin Perinatol 2014;41(1):69-82. http://dx.doi.org/10.1016/jxlp.2013.09.005 [ Links ]
12. Bolisetty S, Dhawan A, Abdel-Latif M, BajukB, Stack J, Lui Κ. Intraventricular hemorrhage and neuro developmental outcomes in extreme preterm infants. Pediatrics 2014;133(1):55-62. http://dx.doi.org/10.1542/peds.2013-0372 [ Links ]
13. Linder N, Haskin O, Levit O, et al. Risk factors for intraventricular hemorrhage in very low birth weight premature infants : A retrospective case- control study. Pediatrics 2003;111(5):e590-e595. https://doi.org/10.1542/peds.lll.5.e590 [ Links ]
14. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde GJ. Research electronic data capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377-381. http://dx.doi.Org/10.1016/j.jbi.2008.08.010 [ Links ]
15. Vermont Oxford Network. Report of very low birth weight infants born in 2015. Burlington: VON, 2016. [ Links ]
16. Cooper PA, Ballot DE, Chirwa P, Ramdin T. Neonatal Protocols. Johannesburg: Charlotte Maxeke Johannesburg Academic Hospital, Neonatal Unit, 2014:27. [ Links ]
17. Sarkar S, Bhagat I, Dechert R, Schumacher RE, Donn SM. Severe intraventricular hemorrhage in preterm infants: Comparison of risk factors and short-term neonatal morbidities between grade 3 and grade 4 intraventricular hemorrhage. Am J Perinatol 2009;26(6):419-424. http://dx.doi.org/10.1055/s-0029-1214237 [ Links ]
 Correspondence:
Correspondence:
A Ghoor
azra.ghoor@gmail.com














