Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Child Health
versión On-line ISSN 1999-7671
versión impresa ISSN 1994-3032
S. Afr. j. child health vol.10 no.1 Pretoria ene./mar. 2016
http://dx.doi.org/10.7196/sajch.2016.v10i1.1099
RESEARCH
An evaluation of the screening for retinopathy of prematurity in very-low-birth-weight babies at a tertiary hospital in Johannesburg, South Africa
Z DadooI; D E BallotII
IMB BCh, FCPaed (SA); Department of Paediatrics and Child Health, University of the Witwatersrand and Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
IIMB BCh, FCPaed (SA), PhD; Department of Paediatrics and Child Health, University of the Witwatersrand and Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Retinopathy of prematurity (ROP) is a leading cause of blindness for very-low-birth-weight (VLBW, <1 500 g) babies. ROP screening identifies babies that require treatment to prevent major visual impairment.
OBJECTIVES: To evaluate the screening for ROP at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) by reviewing the number of babies screened according to the CMJAH guidelines, the grades of ROP found and the treatment modality received.
METHODS: This was a retrospective record review of VLBW babies born between 1 January 2013 and 31 December 2013 at CMJAH, whether inborn or transferred in. The babies were divided into two groups based on age at final outcome. Final outcome was defined as death, discharge or transfer out of the unit. The 'early' outcome group had their final outcome before day 28 oflife. The 'late' outcome group had their final outcome at day 28 or more of life. The early outcome group qualified for outpatient ROP screening and the late outcome group qualified for inpatient ROP screening.
RESULTS: There were a total of 572 VLBW babies at CMJAH during this time period. The babies had a mean birth weight of 1 127 (standard deviation (SD) 244.75) g and gestational age of 29 (2.743) weeks. The mean duration of stay was 29 (21.66) days and there were 309 female babies. Of these 572 babies, 304 comprised the early outcome group and 268 comprised the late outcome group. In the early outcome group babies who were transferred out of the unit or died were excluded; therefore the remaining 147 babies discharged home qualified for outpatient ROP screening. Inpatient ROP screening was carried out in 36/147 (24.4%) of these babies (not in accordance with ROP screening guidelines). ROP was documented in 4/36 (11.1%). Outpatient ROP screening records were unavailable. Exclusions from the late outcome group included five babies. In the late outcome group 111/263 (42.2%) were screened for ROP. ROP was found in 17%. One baby required treatment with intravitreal antivascular endothelial growth factor (VEGF) and three babies required surgery
CONCLUSIONS: More than half of the babies in the late outcome group were not screened during their stay (57.8%). More than one-third of babies were discharged prior to reaching the current recommended age for screening. Efforts need to be intensified to identify and screen all eligible babies prior to discharge. Outpatient ROP screening is not well documented; therefore prevalence cannot be established.
Retinopathy of prematurity (ROP) is known to be an important cause of visual impairment and blindness in the surviving premature population. Over the last 10 - 15 years an estimated 50 000 children are blind as a result of ROP, and it is likely that many more are unilaterally blind or visually impaired.[1] As the disease can be present without any symptoms or clinical signs, it is necessary to screen premature babies for ROP. Most ROP will resolve by itself and only requires continued monitoring until resolution or maturation of retinal vessels occurs. However, severe forms of ROP require treatment to preserve or salvage vision and to improve quality of life. In developed countries two epidemics of blindness due to ROP have been described. The first occurred predominantly in the USA in the 1940s - 1950s. The principal risk factor was the supply of unmonitored supplemental oxygen to the premature baby. The subsequent restriction in oxygen use led to a decrease in blindness due to ROP. The second epidemic started in the 1970s as a result of the higher survival rates of extremely premature babies secondary to advances in neonatal intensive care units (NICUs).[1]
A third epidemic of ROP is currently said to be occurring in middle-income countries[2] such as South Africa (SA). Reasons for this include improved survival of premature babies in these countries, together with a lack of adequate monitoring of oxygen therapy. Countries with infant mortality rates (IMRs) >60/1 000 live births do not usually have NICUs; therefore, premature babies do not survive and these countries have a low incidence of ROP.[2] Countries with IMRs of 9 -60/1 000 live births represent the highest burden of blindness caused by ROP, as more premature babies survive in NICUs where oxygen administration may be poorly monitored.[1] SA's IMR for 2011 was 35/1 000 live births.[3] As we succeed in improving our IMR, strategies need to be in place to target prevention of known risk factors for ROP and screening for ROP that may require treatment.
If screening programmes are not put in place, the incidence of blindness from untreated ROP is likely to increase. It was first reported in 1988 that treatment could improve the outcome for severe ROP. [4] This makes ROP screening a priority. The World Health Organization (WHO)'s Vision 2020 programme has recognised ROP as an important cause of childhood blindness in industrialised and middle-income countries.[5] Their strategies advocate examining premature babies at risk of ROP, treating those premature babies with severe ROP and promoting oxygen monitoring to all premature babies receiving oxygen therapy.
The two important aspects of screening for ROP are who to screen and when to screen them. Knowledge of risk factors for ROP helps to identify who needs to be screened. Risk factors for ROP are divided into two groups - prenatal and postnatal.[6] Prenatal factors include gestational age (GA) and birth weight. Postnatal factors include prolonged exposure to oxygen and other identified markers of neonatal illness severity. Examples of the latter include the need for mechanical ventilation, the presence of sepsis or intraventricular haemorrhage, the administration of blood transfusions and poor postnatal weight gain.[7] Low levels of serum insulin-like growth factor 1 (IGF-1) are found in babies with poor postnatal weight gain.[8] General criteria used in screening programmes are birth weight and GA, combined with sickness criteria.[9] The recommended age for screening is based on the timing of the occurrence of ROP and is related to the maturity of the retinal vessels.
There are concerns that in middle- and low-income countries, compared with high-income countries, a greater number of older and larger babies are presenting with ROP. In a large prospective study done at Chris Hani Baragwanath Academic Hospital to establish the frequency of ROP it was concluded that this was not the case, and the screening weight could safely be lowered to 1 250 g.[10]
According to the latest ROP guidelines published in the South African Medical Journal (SAMJ), all very-low-birth-weight (VLBW) babies <1 500 g or 32 weeks' GA should be screened for ROP.[11] Screening is repeated until retinal vascularisation has reached a stage where the risk of a serious adverse outcome is considered minimal. ROP screening is carried out by an ophthalmologist and by means of indirect ophthalmoscopy. Newer screening techniques involve the use of digital cameras to capture images of the retina. The 2013 SAMJ guidelines recommend screening all VLBW babies at 4 - 6 weeks chronological age or 31 - 33 weeks corrected GA - whichever comes last. The guidelines detail where and how to screen, as well as how to follow up and manage patients and when to stop screening.[11] These guidelines are in line with the WHO Vision 2020 strategy. Vision 2020 ensures the availability of ophthalmologists experienced in indirect ophthalmoscopy who can identify premature babies who require treatment for ROP, that babies at risk for ROP have their fundi examined starting 4 - 6 weeks after birth, and that those with severe disease are treated immediately.[5]
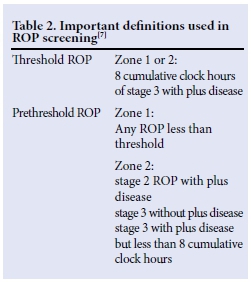
ROP is classified according to the International Classification of ROP (ICROP), was standardised in 1984, and updated in 1987 and again in 2005.[12] ROP is characterised by using four components (Table 1): (i) location (zones 1 - 3); (ii) severity (stages 1 - 5); (iii) extent (circumferential location of the disease reported as clock hours); and (iv) plus disease (tortuosity of posterior retinal vessels).[71 Two important definitions are those of threshold ROP and prethreshold ROP (Table 2). Threshold ROP carries a risk of blindness of 50%, which can be reduced to 25% with treatment. Pre-threshold ROP can require either treatment or close observation - depending on the type. The various treat ment options available for ROP include cryotherapy, laser ablative therapy, intravitreal antivascular endothelial growth factor (anti-VEGF) and retinal reattachment.[7] Not all of these options are available in our setting.
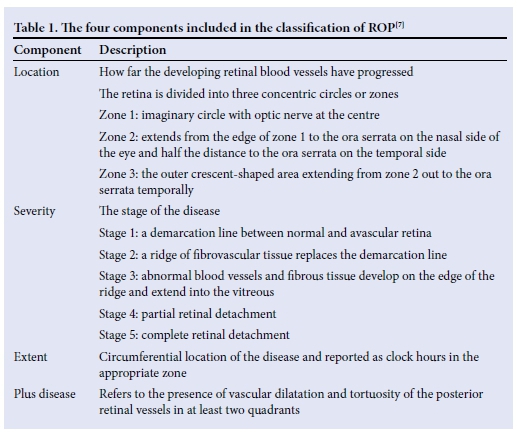
In SA, approximately 1 in 5 of all VLBW babies is affected by ROP. A study at Chris Hani Baragwanath Academic Hospital found the incidence to be ~17%.[10] In another study undertaken at Tygerberg Children's Hospital in Cape Town, the incidence was found to be 21.8%.[13] A study by Delport et al.[14] at Kalafong Hospital, Pretoria, found the incidence of ROP to be 24.5%. The incidence at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) is unknown.
The present study aimed to review the screening programme for ROP in VLBW babies at CMJAH.
Methods
This study was undertaken at CMJAH, a tertiary care institution that serves as a referral centre for the primary care clinics and other hospitals in the area. It was a retrospective record review of all the VLBW babies admitted to CMJAH from 1 January 2013 to 31 December 2013, whether inborn or transferred in. Babies who died or were transferred out before day 28 of life were excluded from the present study.
Patient information was obtained from an existing neonatal VLBW database at CMJAH, which is kept for the purpose of clinical audit purposes. The database consists of standard information that is collected upon the discharge of each baby. Data are managed using REDCap (Research Electronic Data Capture) tools hosted by the University of the Witwatersrand.[15] All definitions in the database are according to the Vermont Oxford Network (VON) (www.vtoxford.org).
The ROP screening guidelines for CMJAH were derived from the SAMJ 2013 ROP screening guidelines, and state that all VLBW babies or those born at a GA <32 weeks should be screened at 4 - 6 weeks chronological age. In babies who were screened more than once, the worst grade of ROP recorded was used for the purpose of the study. Intravitreal anti-VEGF and surgery for ROP were available at CMJAH at the time of the study.
Prior to discharge, all VLBW babies at CMJAH were transferred to kangaroo mother care (KMC) once their current weight was >1 000 g, they were tolerating full enteral feeds and they were off supplemental oxygen. Whenever possible, these babies were transferred to regional hospitals for continuing care. The VLBW babies were discharged from hospital once they had reached a weight >1 600 g, were taking full oral feeds (either by cup or breast) and were maintaining their blood glucose levels. Babies were referred for ROP screening to the ophthalmologist at the discretion ofthe attending paediatric registrar, in accordance with the abovementioned guidelines. Babies in KMC were included in the screening programme. Results of the ROP screening were recorded on the daily charts for each patient.
Groups
The VLBW babies in the study population were divided into two groups based on the calculated chronological age at final outcome, in accordance with the ROP screening guidelines mentioned above. Final outcome was defined as death, discharge or transfer out of the unit. The final outcome for the 'early' outcome group occurred before day 28 of life; in the 'late' outcome group final outcome occurred on day 28 of life or later. The early outcome group qualified for outpatient ROP screening and the late outcome group qualified for inpatient ROP screening.
Statistical analysis
The relevant data for the present study were extracted from the neonatal database and exported to a Microsoft Excel (USA) spreadsheet. Demographic information, outcome, whether ROP screening had been performed and the grade and treatment of ROP (intravitreal anti-VEGF or surgery) were collected for each patient. Duration of stay and chronological age at final outcome (discharge, death or transfer out to a regional hospital) were calculated.
The data from the Excel spreadsheet were imported to the statistical software IBM (USA) SPSS Statistics version 22 for analysis. Data were described using standard statistical methods. Categorical variables were described using frequencies and percentages, and continuous variables by using measures of central tendency - mean and standard deviation - as the data were normally distributed.
Ethics approval
The study was approved by the Committee for Research on Human Subjects, University of the Witwatersrand, Johannesburg (clearance certificate No. M130947).
Results
A total of 572 (309 female) VLBW babies were admitted to the neonatal unit during the study period. A total of 162 babies were excluded. There were 128 deaths and 29 transfers to regional hospitals prior to 28 days. Five babies were transferred in to the unit after 28 days of life for surgical procedures; 2 died immediately and 3 were transferred back to their original hospitals within 2 days. The final sample therefore included 410 babies. The mean birth weight was 1 127 g with a standard deviation (SD) of 244.75 g, and the mean (SD) GA was 29 (2.74) weeks. The mean age at admission was 1 day (5.806) and the babies had a mean duration of stay of 28 (21.66) days. There were 147 babies in the early outcome group and 263 babies in the late outcome group (Fig. 1).
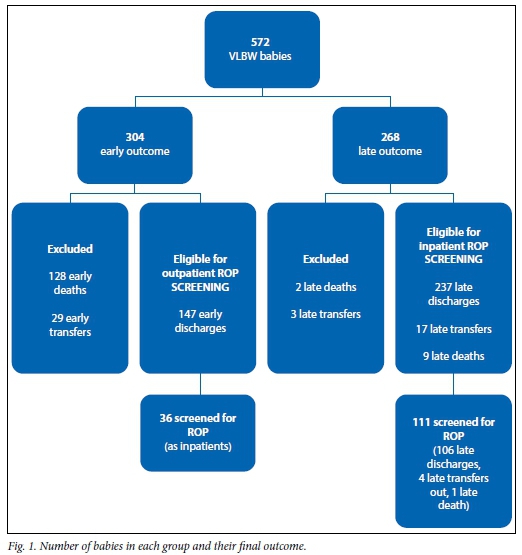
ROP screening was documented in 147/410 (35.9%) VLBW babies. The ROP findings are summarised in Table 3. Plus disease was not found in any of the babies. Intravitreal anti-VEGF treatment was used in one baby and surgical treatment was documented in three babies.
Although the 147 babies in the early outcome group were not required to be screened as inpatients, ROP screening was carried out in 36 (24.5%) and of these 4 (11.1%) had evidence of ROP. Screening for ROP was undertaken in 111/263 (42.2%) babies in the late outcome group and 19 (17.1%) had evidence of ROP.
Discussion
This study shows that less than half of the VLBW babies at CMJAH eligible for inpatient ROP screening according to the hospital's guidelines were actually screened for ROP.
More than one-third of babies were discharged before they had reached the required age for screening. Despite this, 24.5% of these babies were screened before 4 weeks of life. Of concern is that 11.1% of these babies had ROP. This group of early discharges is important as they require outpatient ROP follow-up. It is not known whether these babies attended screening for ROP as outpatients, so it is possible that a number of babies with ROP were missed. Education of caregivers in this group is essential, as defaulters to follow-up are at risk of presenting with more severe grades of ROP and increased morbidity.
Although a true prevalence for ROP at CMJAH for 2013 cannot be calculated, as a result of the low level of screening, ROP was found in 15.6% of VLBW babies, which is similar to the 17% rate reported at Chris Hani Baragwanath Academic Hospital.[10] Other SA studies reported slightly higher rates - 21.8% at Tygerberg Children's Hospital [13] and 24.5% at Kalafong Hospital.[14]
This review shows that the inpatient ROP screening at CMJAH is not optimal and needs to be improved. Inpatient ROP screening was not carried out in 57.8%. Babies at risk need to be promptly identified. The attending medical staff (interns, medical officers, registrars and consultants) should to be familiar with the guidelines. Junior staff will need to be educated on the harms of oxygen therapy and the subsequent complication of ROP and its consequences. Although it may seem attractive to delay the discharge of VLBW babies until they have achieved the recommended age for ROP screening, this is not feasible because of high patient numbers and extreme pressure for beds.
Adjusting the screening protocol to allow ROP screening at a younger age in those babies who will be discharged before 28 days of age would be a simpler solution and would prevent missed opportunities to identify babies with ROP.
No babies were recorded to have plus disease. These data may have not been captured on discharge, were truly not present or may have been under-reported by the ophthalmologist performing the screening. Bigger babies are also at risk of ROP. The 2013 SAMJ ROP guideline suggests that premature babies with weights between 1 500 g and 2 000 g may also be at risk if they have risk factors; if oxygen monitoring in this group of babies has been suboptimal then screening should be considered.[11] This group of babies was not included in the present study, but should not be overlooked in screening programmes for ROP.
Ideally, an electronic prospective data capture system needs to be implemented to capture all the results of ROP screening - both inpatient and outpatient. This would only be possible in conjunction with the Department of Ophthalmology, especially regarding the outpatient screening. This will assist greatly with future research and in gauging the incidence of ROP at CMJAH.
Study limitations
One limitation is the design of the study - the retrospective nature of the study means a precollected dataset was used. ROP information is not available for babies on the low-birth-weight (LBW) database who may have a GA of <32 weeks but a weight of >1 500 g. Another potential limitation is that of inter-observer error in classifying the grade of ROP present, as different ophthalmology registrars did the screening, with different levels of skill and experience.
Conclusion
More than half of VLBW babies who met criteria for ROP screening according to CMJAH ROP screening guidelines were not screened during their inpatient stay. Efforts need to be intensified to identify these babies and screen them prior to discharge.
Records for outpatient ROP screening are not well organised and not easily accessible at both the neonatal follow-up clinic and the ophthalmology unit. There is a need for a co-ordinated database between the two specialties. In this regard, a true prevalence for ROP at CMJAH cannot be established.
Screening for ROP should include all babies with a GA of <32 weeks (even if their weight is >1 500 g). In addition, babies weighing between 1 500 g and 2 000 g with risk factors for ROP should not be omitted from screening programmes.
References
1. Gilbert C. Retinopathy of prematurity: A global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev 2008;84(2):77-82. [http://dx.doi.org/10.1016/j.earlhumdev.2007.11.009] [ Links ]
2. Zin A, Gole G. Retinopathy of prematurity - incidence today. Clin Perinatology 2013;40(2):185-200. [http://dx.doi.org/10.1016/j.clp.2013.02.001] [ Links ]
3. The World Bank. Infant mortality rate (per 1 000 live births). http://data.worldbank.org/indicator/SP.DYN.IMRT.IN (accessed 11 September 2013). [ Links ]
4. Cryotherapy for retinopathy of prematurity cooperative group. Multicentre trial of cryotherapy for retinopathy of prematurity: Preliminary results. Arch Ophthalmol 1998;106(4):471-479. [http://dx.doi.org/10.1001/archopht.1988.01060130517027] [ Links ]
5. World Health Organization and the International Agency for the Prevention of Blindness Joint Initiative. Vision 2020: The Right to Sight Action Plan 2006-2010. http://www.iapb.org/vision-2020/what-is-avoidable-blindness/childhood-blindness (accessed 11 September 2013). [ Links ]
6. Wikstrand M, Hard A, Niklasson A, Smith L, Lofqvist C, Hellstrom A. Maternal and neonatal factors associated with poor early weight gain and later retinopathy of prematurity. Acta Paediatr 2011;100(12):1528-1533. [http://dx.doi.org/10.1111/j.1651-2227.2011.02394.x] [ Links ]
7. Cloherty JP, Eichenwald EC, Hansen AR, Stark AR. Manual of Neonatal Care. 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2012:840-845. [ Links ]
8. Smith L, Hard AL, Hellstrom A. The biology of retinopathy of prematurity. Clin Perinatology 2013;40(2):201-214. [http://dx.doi.org/10.1016/j.clp.2013.02.002] [ Links ]
9. Holmstrom G, Hellstrom A, Jakobsson P, Lundgren P, Tornqvist K, Wallin A. Swedish national register for retinopathy of prematurity (SWEDROP) and the evaluation of screening in Sweden. Arch Ophthalmol 2012;130(11):1418-1424. [http://dx.doi.org/10.1001/archophthalmol.2012.2357] [ Links ]
10. Mayet I, Cockinos C. Retinopathy of prematurity in South Africa at a tertiary hospital: A prospective study. Eye (Lond) 2006;20(1):29-31. [http://dx.doi.org/10.1038/sj.eye.6701779] [ Links ]
11. Visser L, Singh R, Young M, Lewis H, McKerrow N (ROP working group South Africa). Guideline for the prevention, screening and treatment of retinopathy of prematurity (ROP). S Afr Med J 2013;102(2):116-125. [http://dx.doi.org/10.7196/samj.6305] [ Links ]
12. International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol 2005;123(7):991-999. [http://dx.doi.org/10.1001/archopht.123.7.991] [ Links ]
13. Van der Merwe S, Freeman N, Bekker A, Harvey J, Smith J. Prevalence of and risk factors for retinopathy of prematurity in a cohort of preterm infants treated exclusively with non-invasive ventilation in the first week after birth. S Afr Med J 2013;103(2):96-100. [http://dx.doi.org/10.7196/samj.6131] [ Links ]
14. Delport SD, Swanepoel JC, Odendall PJL, Roux P. Incidence of retinopathy of prematurity in very low birth weight infants born at Kalafong Hospital, Pretoria. S Afr Med J 2002;92(12):986-990. [ Links ]
15. Harris PA, Taylor R, Thielke R, Payne R, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;43(2):377-381. [http://dx.doi.org/10.1016/j.jbi.2008.08.010] [ Links ]
 Correspondence:
Correspondence:
Z Dadoo
drzdadoo@gmail.com














