Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.10 n.1 Pretoria Jan./Mar. 2016
http://dx.doi.org/10.7196/sajch.2016.v106i1.835
RESEARCH
Prevalence of positive coeliac serology in a cohort of South African children with type 1 diabetes mellitus
S TayobI, II; K PillayII, III; B TlouIV; Y GanieI, II
IMB ChB, DCH, FCP (Paed), Cert Paed Endo; Department of Paediatrics and Child Health, Nelson R Mandela School of Medicine, Faculty of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IIMB ChB, DCH, FCP (Paed), Cert Paed Endo; Division of Paediatric Endocrinology, Inkosi Albert Luthuli Central Hospital, Durban, South Africa
IIIMB ChB, DCH, FCP (Paed), Cert Paed Endo; Centre for Paediatric Endocrinology and Diabetes, Westville Hospital, Durban, South Africa
IVMSc; Discipline of Public Health Medicine, School of Nursing and Public Health, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: Coeliac disease (CD) is characterised by immune-mediated damage to the mucosa of the small intestine. Both CD and type 1 diabetes (T1D) have common auto-immune origins. Many patients with CD and T1D are asymptomatic or present with only mild symptoms; hence early diagnosis may only be facilitated by serological screening. Distal duodenal biopsy remains the gold standard for confirming the diagnosis.
OBJECTIVE: To describe the prevalence of CD in T1D patients presenting to the paediatric endocrine service at Inkosi Albert Luthuli Central hospital (IALCH) in Durban and document the relationship between positive coeliac serology and small-bowel biopsy results.
METHODS: A retrospective chart review was done at IALCH, the paediatric tertiary referral centre for KwaZulu-Natal (KZN) Province. The study sample included all patients with newly diagnosed T1D diagnosed between January 2008 and December 2011.
RESULTS: A total of 120 newly diagnosed T1D patients were included in the study, of whom 49 (40.8%) were coeliac serology positive and 61 (50.8%) serology negative. There was no significant difference between the two groups regarding mean age of presentation with diabetes, race, sex, urban v. rural origin and baseline anthropometric measurements. Of patients in the serology-positive group, 97.6% had no symptoms suggestive of CD. Of the 49 patients who were coeliac serology positive, 8 (16%) were biopsied: 3 (37.5%) were positive, 1 (12.5%) had intra-epithelial lymphocytes and 4 (50%) were negative. There was a strong positive correlation between biopsy results and titres of endomysial antibody results (p=0.047).
CONCLUSION: There is a high prevalence of coeliac serology positivity in newly diagnosed T1D patients in KZN. This study provides evidence for screening of children with T1D for CD, and also confirms the low prevalence of symptoms.
Coeliac disease (CD) is characterised by immune-mediated damage to the mucosa of the small intestine. These changes are triggered by ingestion of gluten and related substances found in cereal grains. CD affects both developing and developed nations.
CD and type 1 diabetes (T1D) have common auto-immune origins. Both are associated with major histocompatibility complex class II antigen DQ2 encoded by alleles DQA1*501 and DQB1*201, thus providing a common genetic basis for the disease expression.[1] Recent work has also revealed seven shared non-human leukocyte antigen loci associated with CD and T1D.[1] This shared genetic basis is strongly suggestive of a common aetiology for both conditions.[1]
Most estimates put the prevalence of CD at close to 1% of the general population.[1] The prevalence of CD in T1D has been reported to be five to seven times greater than in the general population, with increased prevalence rates among most ethnic groups.[1] Early epidemiological studies showed that CD was largely a disorder of white populations; however, recent studies reveal a similar prevalence in other race groups.
Although sampling rates and diagnostic criteria differ among studies, rates of biopsy-proven CD in paediatric T1D range from as low as 2.4% in Finland to 16.4% in Algeria.[1] The prevalence of biopsy-proven CD in the paediatric T1D population was 10.3% in a Libyan study, 4% in an Egyptian study and 5.3% in a Tunisian study.[1] The prevalence of CD in sub-Saharan Africa (which includes different ethnic populations to other parts of Africa) is as yet undescribed.
The classic presentation of CD includes symptoms related to gastrointestinal malabsorption, such as malnutrition, failure to thrive, diarrhoea, anorexia, constipation, vomiting, abdominal distension and pain. Non-gastrointestinal symptoms of CD include short stature, pubertal delay, fatigue, vitamin deficiencies and iron-deficiency anaemia.[1] Many patients with T1D who have CD are either asymptomatic (silent disease) or present with only mild symptoms.[1] Hence early diagnosis may only be facilitated by serological screening.
The main reason for screening asymptomatic individuals is to institute early treatment, thereby decreasing the risk of long-term CD-related complications, including malignancy of the gastrointestinal tract and osteoporosis, iron-deficiency anaemia and growth failure secondary to malabsorption.[1-3] A second advantage of screening asymptomatic patients with diabetes is the potential for improved diabetes control; undiagnosed CD has been associated with increased frequency of hypoglycaemic episodes.[3] Longstanding CD may be associated with an increased risk of retinopathy, and non-adherence to a gluten-free diet may increase the risk of microalbuminuria.[3]
Intestinal biopsy is considered the gold standard in the diagnosis of CD. The staging of mucosal changes suggested by Marsh is now widely used.[4]
The introduction of serological testing has facilitated screening of populations at risk for CD. Screening tests for IgA endomysial antibodies (EMA) and tissue transglutaminase (tTG) IgA have been reported to be the most sensitive and specific.[1] Both tests demonstrate sensitivity and specificity of >90%.[3,5,6] According to the American Gastroenterology Association[7] in the primary care setting the tTG IgA is the gold standard serological test for the detection of CD.
CD and autoimmune thyroid disorders share a common genetic predisposition, namely the DQ2 allele, which accounts for the higher incidence of thyroid autoimmune disorders in CD than in the general population.™
Despite the advent of sensitive and specific serological testing, routine screening for CD in diabetic populations may not be universal practice. The International Society for Pediatric and Adolescent Diabetes (ISPAD) and the European Society for Pediatric Gastroenterology and Hepatology (ESPGHAN) both recommend screening of T1D patients for CD.[3,9] ISPAD recommends screening for CD at the time of diabetes diagnosis, and every 1 - 2 years thereafter.
From 2008 onwards all new T1D patients attending the Inkosi Albert Luthuli Central Hospital (IALCH) diabetes clinic in Durban have been screened for CD at diagnosis. Analysis of these data to assess the prevalence of CD in this T1D population will provide information on implications for future screening practices.
Methods
The study design was a retrospective chart review. The study population included all children with newly diagnosed diabetes referred to the Paediatric Endocrine Unit at IALCH between January 2008 and December 2011. Children and adolescents with type 2 diabetes (T2D) or neonatal diabetes or known T1D patients with CD were excluded.
The study sample was divided into two groups based on their coeliac serology results and then compared. TTG IgA and IgG, EMA and antigliadin antibody (AG) IgA and IgG were measured in all patients. The Bio-Rad Laboratories (South Africa) kit was used for the tTG and AG enzyme-linked immunosorbent assay and the Diagnostic Technical Services (South Africa) kit was utilised for the EMA indirect immunofluorescence. A positive tTG IgA or IgG (>15 U/mL), EMA or AG IgA or IgG (>15 U/mL) were included in the analysis. Serological tests and biopsies were carried out while patients were still on a gluten-containing diet. Total IgA was not routinely measured but requested only in those with an initial negative serological result in whom there was a strong suspicion of disease.
Symptoms were captured as either present or absent using a standardised questionnaire. Specific symptoms included were chronic diarrhoea, constipation, abdominal pain, nausea, vomiting, flatulence, bloating, loss of weight and fatigue.
Data were collated on an Excel (Microsoft, USA) spreadsheet and analysed using SPSS version 21 (IBM, USA). Categorical variables were assessed using the Pearson's χ2 test and quantitative variables using the Student's t-test. A p-value of <0.05 was considered statistically significant.
Results
There were 139 newly diagnosed diabetes patients referred to the paediatric endocrine service between January 2008 and December 2011. Of these, 120 (86.3%) had T1D, 8.6% had T2D and 5% had neonatal diabetes. Of the 120 T1D patients, 49 (40.8%) were coeliac serology positive, 61 (50.8%) were coeliac serology negative, and the serological result was unknown in 10 patients. Hence the prevalence of positive coeliac serology in our cohort of T1D patients was 44.5%. Of the 49 patients with a positive serological result, 17 were tTG IgA positive and all of the 8 patients who underwent biopsy were tTG IgA positive.
The coeliac serology-positive and -negative groups were compared in terms of their demographic features, as summarised in Table 1. The mean age at presentation was 7.32 years in the serology-positive group and 6.84 years in the serology-negative group (p=0.464).
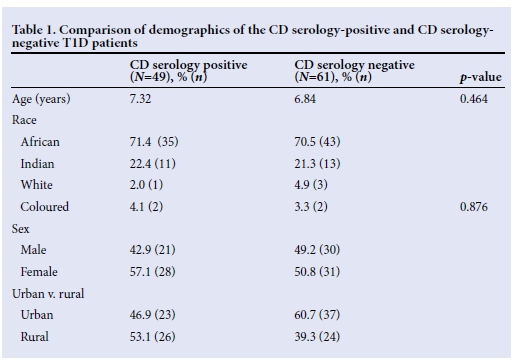
The majority of patients in both groups were black Africans (71.4% in the serology-positive group and 70.5% in the serology-negative group). In the serology-positive group 22.4% of the patients were Indian, 2% were white and 4.1% were coloured. In the serology-negative group 21.3% of the patients were Indian, 4.9% were white and 3.3% were coloured. Ethnicity had no significant effect on the prevalence of positive coeliac serology (p=0.876).
There was a female predominance overall which was more marked in the coeliac serology-positive group, in which 57.1% were female and 42.9% were male; however, this was not statistically significant (p=0.509).
The majority of the patients in the serology-positive group were of rural origin (53.1%), while in the serology-negative group 60.7% of patients were of urban origin. This difference was not significant (p=0.151).
There was no significant difference observed in the baseline anthropometric measurements between the two groups.
It was interesting to note that in the serology-positive group 97.6% (n=41) of patients had no symptoms suggestive of CD, while only 2.4% (n=1) had symptoms of CD.
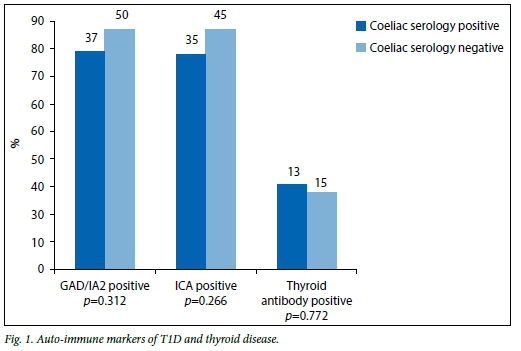
The auto-immune markers of T1D as well as the thyroid antibodies in both the coeliac serology-positive and -negative groups were compared (Fig. 1). Of patients in the serology-positive group 77.1% (n=37) had positive glutamic acid decarboxylase (GAD) or insulin auto-antibodies 2 (IA2), while 85% (n=50) of patients in the serology-negative group had positive GAD or IA2 (p=0.312). Of the patients, 76% (n=35) in the serology-positive group and 85% (n=45) in the serology-negative group had positive islet cell antibodies (ICA) (p=0.266), while 29% (n=13) of patients in the serology-positive group and 26% («=15) in the serology-negative group had positive thyroid antibodies (p=0.772).
Of the 49 coeliac serology-positive patients, eight (16%) underwent biopsy. This is a very small number and is one of the limitations of this study. Three of the 8 patients (37.5%) had a positive biopsy result, 1 (12.5%) had intra-epithelial lymphocytosis and 4 (50%) had negative biopsies. Of the 3 positive biopsies, 1 was classified as Marsh stage 1, and 2 as Marsh stage 3a. All of the eight patients who were biopsied were tTG IgA positive. Two of the three positive biopsies were also EMA positive. Three of the five biopsy-negative patients were positive for all three antibodies, one was EMA and tTG IgA positive (AG IgA negative) and one was only tTG IgA positive.
Although the number of patients biopsied was inadequate, the results showed a positive correlation (p=0.047) between biopsy and the presence of EMA. A correlation could not be assessed for tTG IgA as all of the eight patients who underwent biopsy had a positive tTG IgA. There was no significant correlation between biopsy and tTG IgG or AG.
Discussion
The prevalence of positive coeliac serology in our cohort of T1D patients was 44.5%. If the TTG IgA alone had been used, the prevalence in this study would have decreased to 15.5%. This is a high prevalence compared with previously described rates for other countries. However, our prevalence rates were for coeliac serology positivity, while the others were for biopsy-proven CD. A limitation and contributing factor to the high prevalence of positive coeliac serology in this study is that patients with positive AG IgA were also included in the analysis. Combining several tests for CD in lieu of tTG IgA alone may marginally increase sensitivity but reduces specificity and is therefore not recommended. The specificity of coeliac antibody testing in black South Africans is not known. A control group of non-diabetic black South Africans was not used to compare the serological results. Causes of false-positive serology results, such as inflammatory bowel disease, irritable bowel syndrome, cirrhosis and viral infections, were excluded.
There may have been a racial bias as the majority of patients were of black African origin in both the coeliac serology-positive (71.4%) and -negative (70.5%) groups; however, this reflects the population statistics in South Africa (SA). The research was conducted in a government hospital, which may also result in possible bias as private-sector patients, who represent a different demographic and socioeconomic group, were not included.
The mean age of biopsy-proven CD in T1D in an Italian study of children in 2008 was 8.1 (4.3) years.[1] The mean age of the serology-positive patients was similar in this study at 7.32 years. This is reflective of the shift toward a younger age of onset of T1D worldwide.
This study found higher coeliac serology positivity among females (57.1%) than males (42.9%), which is consistent with international data.
Of patients in the serology-positive group, 97.6% had no symptoms of CD. This was a retrospective study, and it is likely that there was inadequate information in the patient records, which may explain the high percentage of asymptomatic patients compared with other studies. Our result was higher than that reported in a North American study performed at a CD clinic, which revealed that 71.4% of children reported no gastrointestinal symptoms at the time of a positive screening test.[1] A possible reason for our results is that the predominance of gastrointestinal symptoms is most common in children <3 years,[1] and the mean age of our serology-positive group was 7.32 years. In asymptomatic individuals at high risk (e.g. those with T1D) CD should always be diagnosed using duodenal biopsies, as this population more often have false-positive tTG results.[9] Biopsy may not be required in those patients with signs or symptoms suggestive of CD and tTG levels >10 times the upper limit of normal.[9]
There was no significant difference between the two groups in terms of the auto-immune markers of T1D or thyroid antibodies. This is in keeping with a study by Sumnik et al., cited by Fasano,[10] who performed a multicentre retrospective case-control study comparing data from 84 diabetic children with CD with 167 diabetic children without CD, and concluded that the occurrence of thyroid auto-immunity in diabetic children is not related to coexisting CD. However, Velluzi et al., cited by Kumar et al.[8]found that CD patients had a three- to fourfold increase in the incidence of thyroid auto-immunity.
Of the 49 coeliac serology-positive patients, 8 (16%) underwent biopsy. This is a small number and a significant limitation of the study. There is no qualified paediatric gastroenterologist at IALCH and it was a challenge to obtain biopsies. Of the 8 patients biopsied, 3 (37.5%) had a positive result (1 Marsh stage 1 and 2 Marsh stage 3a). This is a lower rate than expected. A possible explanation for this result is that the biopsy technique was not standardised. The biopsies were not performed by the same doctor (paediatric surgeon or adult gastroenterologist), and the number of specimens obtained and biopsy sites differed. At least four biopsies should be taken to increase sensitivity and specificity as the typical coeliac lesions can be patchy.[4,8] Correct histological interpretation by the pathologist is another important issue. Specimens were not assessed by the same pathologist; however, all specimens were subsequently reviewed by the head of the Department of Pathology.
Although the number of patients biopsied was small, the positive correlation (p=0.047) between biopsy and EMA results found in this study was in keeping with a review article which examined 32 studies and found a mean specificity and sensitivity of 99% and 95% respectively for this serological marker.[1] The same article found the mean specificity and sensitivity of tTG IgA in 27 studies to be 95% and 87% respectively.[1] However, a correlation for tTG IgA could not be assessed in this cohort as all patients who underwent biopsy had a positive tTG IgA result. Numerous studies have documented the poor accuracy of the AG IgA tests, making them unsuitable for screening purposes.
Study limitations
This study took the form of a retrospective chart review. Patients with a positive AG IgA result were included in the analysis, resulting in a higher than expected prevalence of positive coeliac serology. Specific symptom variables were not individually captured, and this may explain the low prevalence of symptoms in patients. An important limitation is that only a small number of patients were biopsied and that the biopsy procedure was not standardised.
Conclusion
There is a high prevalence of coeliac serology positivity in newly diagnosed T1D patients in KwaZulu-Natal Province, SA. Although all patients did not undergo biopsy, this study provides evidence for screening of children with T1D for CD. There was no significant difference with respect to GAD/IA2 and ICA or thyroid antibodies between the two groups. The relationship between CD and glycaemic control in these patients remains to be established. A prospective follow-up study is required where patients are commenced on a gluten-free diet and clinical and glycaemic outcomes are assessed.
Recommendations
ISPAD recommends screening for CD using the TTG IgA and/or the EMA in all T1D patients at diagnosis and every 1 - 2 years thereafter. The ESPGHAN recommends the use of TTG IgA as the initial screening test for CD.[91 All three coeliac antibodies should not be routinely measured for screening purposes. We support the ISPAD recommendations to screen all newly diagnosed T1DM for CD in SA. Patients with a positive screening test should be referred to a paediatric gastroenterologist for a duodenal biopsy to confirm the diagnosis of CD.
References
1. Sud S, Marcon M, Assor E, Palmert MR. Coeliac disease and paediatric type 1 diabetes: Diagnostic and treatment dilemmas. Int J Paediatr Endocrinol 2010;2010:161285. [http://dx.doi.org/10.1155/2010/161285] [ Links ]
2. Cronin CC, Shanahan F. Insulin dependent diabetes mellitus and coeliac disease. Lancet 1997;349(9058):9058. [http://dx.doi.org/10.1016/S0140-6736(96)09153-2] [ Links ]
3. Kordonouri O, Klingensmith G, Knip M, et al. Other complications and diabetes-associated conditions in children and adolescents. Pediatr Diabetes 2014;15(Suppl. 20):S270-278. [http://dx.doi.org/10.1111/pedi.12183] [ Links ]
4. Sollid LM, Lundin KEA. Diagnosis and treatment of coeliac disease. Mucosal Immunol 2009;2(1):3-7. [http://dx.doi.org/10.1038/mi.2008.74] [ Links ]
5. Lewis NR, Scott BB. Systematic review: The use of serology to exclude or diagnose coeliac disease (a comparison of endomysial and tissue transglutaminase antibody tests). Alimentary Pharmacol Ther 2006;24(1):47-54. [http://dx.doi.org/10.1111/j.1365-2036.2006.02967.x] [ Links ]
6. Hill ID. What are the sensitivity and specificity of serologic tests for celiac disease? Do sensitivity and specificity vary in different populations? Gastroenterology 2005;128(4 Suppl 1):S25-32. [ Links ]
7. American Gastroenterology Association (AGA). AGA institute medical position statement on the diagnosis and management of coeliac disease. Gastroenterology 2006;131(6):1977-1980. [ Links ]
8. Kumar V, Rajadhyaksha M, Wortsman J. Coeliac disease-associated autoimmune endocrinopathies. Clin Diagn Lab Immunol 2001;8(4):678-685. [ Links ]
9. Husby S, Koletzko S, Korponay-Szabo IR. European Society for Paediatric Gastroenterology, Hepatology, and Nutrition Guidelines for the management of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54(1):136-160. [http://dx.doi.org/10.1097/MPG.0b013e31821a23d0] [ Links ]
10. Fasano A. Systemic auto-immune disease in coeliac disease. Curr Opin Gastroenterol 2006;22(6):674-679. [http://dx.doi.org/10.1097/01.mog.0000245543.72537.9e] [ Links ]
 Correspondence:
Correspondence:
S Tayob
shafeekat@gmail.com














