Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.9 n.4 Pretoria Nov. 2015
http://dx.doi.org/10.7196/sajch.2015.v9i4.925
ARTICLE
Immunisation and vitamin A capsule coverage in a semi-urban area of KwaZulu-Natal Province, South Africa
V ComleyI; N NkwanyanaII; A CoutsoudisIII
IMB BCh, FCPaed (SA); Department of Paediatrics and Child Health, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIMSc (Statistics); Public Health Medicine, University of KwaZulu-Natal, Durban, South Africa
IIIPhD; Department of Paediatrics and Child Health, Nelson R Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: The Expanded Programme on Immunisation (EPI) in South Africa (SA) has had a large effect on vaccine-preventable illnesses, yet there is little in the literature describing access to and utilisation of the programme beyond 1 year of age. Coverage of vitamin A supplementation is examined through District Health Information System data, but this does not give a fair assessment of the lifetime coverage in a child or provide any correlation with the immunisation status of the child.
OBJECTIVES: To describe utilisation and dropout rate with the vitamin A and immunisation programmes over the first 6 years of life among children aged 6 - 8 years in a semi-urban population in KwaZulu-Natal (KZN) Province, SA. A secondary objective was to investigate whether access and dropout rates are associated between these two programmes.
METHODS: A retrospective cohort analysis was performed on 923 anonymised Road-to-Health cards, extracting information on immunisation and vitamin A coverage.
RESULTS: Overall, 92.9% (95% confidence interval (CI) 91.2 - 94.6) and 88.5% (95% CI 86.4 - 90.5) of children were fully immunised by 12 months and 18 months of age, respectively. The percentage of children fully immunised by 6 years of age dropped to 44% (95% CI 41.2 - 47.6). The dropout rates for measles, and diphtheria, pertussis and tetanus 1 - 3 vaccination were 2.4% and 1.2%, respectively. Vitamin A had an overall coverage of 34.9% during 6 - 60 months of life for this population, with children receiving, on average, three doses (interquartile range 2 - 5).
CONCLUSION: Despite good immunisation coverage in the first 18 months of life, there was relatively poor vitamin A coverage, suggesting a need for re-evaluation of the current vitamin A capsule distribution programme.
Several public health programmes targeting children have been introduced in developing countries in order to increase efforts to prevent disease and poor health. Two such well-established programmes, recommended by the World Health Organization (WHO) to reduce morbidity and mortality are the Expanded Programme on Immunization (EPI) and the vitamin A supplementation programme.
The EPI was established in 1974 by the WHO,[1] with the outcome goal that by 2010, 90% of <1-year-olds should receive routine immunisations nationally, and that each district should achieve 80% coverage rates.[2] The South African (SA) EPI came into effect in 1995, with updates occurring in 2009 to include pneumococcus and rotavirus vaccines. The national coverage for 2010/2011 was 86.7%,[3] below the 90% target set by the WHO, which had been reached during 2008 and 2009. Only 30 of the 52 districts in SA reached the target of 80% coverage set out by the WHO.[3]
The vitamin A supplementation programme was introduced by the WHO and international agencies to combat the effects of vitamin A deficiency in children, viz. blindness, impaired functioning of the immune system, increased risk of diarrhoea and measles and the associated increased number of childhood deaths.[4] A systematic review and meta-analysis published in 2011 reported that vitamin A supplementation was associated with a 24% reduction in childhood mortality and morbidity, including blindness.[5] Vitamin A supplementation has therefore, for the last one or two decades, been promoted as a cost-effective strategy to improve childhood outcome with WHO guidelines recommending high-dose vitamin A for infants and children aged 6 - 59 months in settings where vitamin A deficiency is a public health concern.[6] In 2003 SA implemented a policy of routine supplementation to children aged 6 - 59 months every 6 months and to postpartum women.[6] The utilisation of this programme has not been well documented and there has been considerable discussion on the most appropriate means of correcting vitamin A deficiency, and whether alternative strategies to high-dose vitamin A capsules should be considered.
While the vitamin A schedule calls for supplementation every 6 months, the EPI has a different schedule with vaccine delivery at birth, 6 weeks, 10 weeks, 14 weeks, 9 months, 18 months and 6 years, so that delivery of the two programmes only overlaps at 18 months.
SA's biggest source of data on immunisation is derived from data collected by the DHIS (District Health Information System), which only supplies information during infancy (<1 year of age). Therefore, the primary objective of this study was to determine access throughout the immunisation schedule and not just during the infancy period. The second objective of this study was to determine the level of utilisation of the vitamin A programme and to investigate whether access and dropout rates in these two programmes are associated.
Methods
Study population
This Immunisation and Vitamin A Coverage (IVAC) study is an ancillary study linked to the Asenze study of children and their primary caregivers in the Valley of a Thousand Hills, part of the Outer West eThekwini district, KwaZulu-Natal (KZN) Province, SA. The population is of Zulu ethnic background, living in five local tribal authorities and municipal wards, and numbers ~67 000. Children in these communities are at high risk of missing opportunities for preventive health services. Most of the families live in adverse socioeconomic conditions with low levels of formal education and high levels of unemployment, and are simultaneously experiencing the effects of the HIV epidemic in KZN. Although the community is well serviced with seven primary healthcare clinics and mobile clinics throughout the five wards, the physical terrain nevertheless makes access difficult.
The objective of the Asenze study was to determine the prevalence of neuro-developmental disability and identify potential health, contextual and psychosocial predictors of disability among children living in this semi-urban area in KZN.[7,8] During years 2008 - 2010, the Asenze study (phase 1) recruited children aged 4 - 6 years, through a door-to-door survey. Phase 2 follow-up was done 18 - 24 months later, at which time the Road-to-Health cards (RTHCs) of the children were copied. The IVAC study data were extracted from copies of RTHCs that had been used in the parent Azenze study.
Sampling
The RTHCs of this cohort of children were used as source data for this study. Of the 1 078 children's RTHCs copied, data were only extracted from 938 owing to poor copy quality.
Of these 938 cards, 923 were included in the study, with 15 cards being excluded because they were identified as duplicates. Of the 923 cards, 917 had information on immunisation and 653 had records concerning vitamin A supplementation.
The reason for the large discrepancy in the number with vitamin A information compared with immunisation is that in the parent study, the page containing vitamin A records was not initially photocopied; this portion was only copied after interest was shown in this study.
RTHCs were used to collect data on date of birth and sex of the child, vitamin A supplementation status, immunisation status according to the EPI schedule and any out-of-stock vaccines.
Statistical analysis
Data were collected and entered into a Microsoft (USA) Excel spreadsheet and analysed in SPSS version 22 (IBM, USA) and Microsoft Excel.
Proportions with two-sided 95% confidence intervals (CIs) for immunisation dropout rates, and immunisation coverage at 12 months, 18 months and 6 years, were computed.
McNemar's χ2 test was used to test for association between reception of full immunisation and vitamin A supplementation. The significance level was set at p<0.05.
Ethical considerations
Ethics approval was obtained from the Bio-medical Research Ethics Committee of the University of KwaZulu-Natal (Ref: BREC 267/12).
All RTHCs were anonymised prior to viewing and data collection, and there was no direct interaction between the study population and the investigators.
Results
Immunisation
Of the 923 RTHCs analysed, 49.3% were for male children and 917 had information regarding immunisation. The percentage of children who had received full immunisation at 12 months, 18 months and 6 years, as well as percentages of children who received the oral polio vaccine (OPV) 5 antigen, diphtheria and tetanus (DT) vaccine, measles immunisations and diphtheria, pertussis and tetanus (DPT) 1 - 3 immunisation are presented in Table 1.
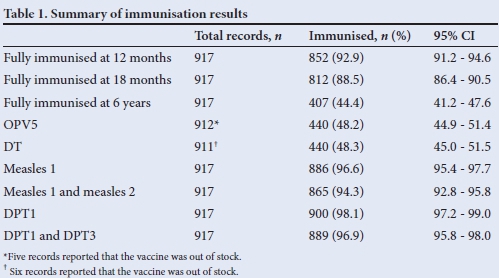
Results in Table 1 depict that the dropout rates for measles 1 and 2, and for DPT1 - 3, were 2.4% and 1.2%, respectively.
Vitamin A
Of the 923 cards analysed, 653 contained information regarding vitamin A doses. The highest level of coverage for vitamin A administration was at 6 months, when 408 of the 653 children (62.5%, 95% CI 58.7 - 66.2%) received vitamin A. This figure gradually decreased to 69 of the 653 children (10.6%, 95% CI 8.2 - 12.9%) receiving a vitamin A dose at 5 years.
Twenty-four of 653 RTHCs (3.7%, 95% CI 2.3 - 5.1%) had no recorded doses of vitamin A. Of these 24 children, 23 were fully immunised at 1 year of age (95.8%, 95% CI 87.7 -100%), 22 were fully immunised at 18 months of age (91.7%, 95% CI 80.6 -100%) and 11 had received all vaccines in the EPI at 6 years (45.8%, 95% CI 25.8 - 65.7%).
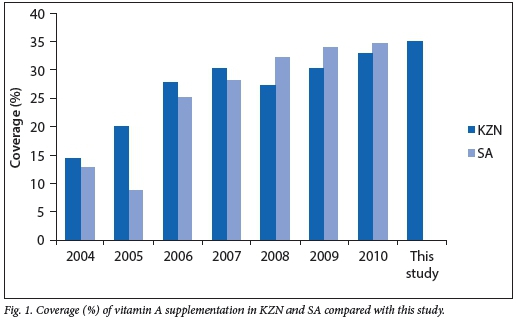
Vitamin A coverage as defined by the DHIS is two doses of vitamin A within 1 year.[9] The percentage of children who received full dosage of vitamin A decreased gradually as the children grew older. Specifically, the percentages of children who had received the full dosage of vitamin A at 12 months, 18 months and 5 years of age were 40.9%, 29.1% and 0.9%, respectively. Over the lifetime of the children in this study population, a total of 2 280 doses of vitamin A were given to all babies during the study period; therefore vitamin A coverage was 34.9%. The average number of doses for each child was three (interquartile range 2 - 5) doses within the age period of 6 -60 months. The children in this study were compared with DHIS data spanning 2004 - 2010, as the study children were born between 2003 and 2005 and the youngest child in the study would have been 5 years of age by 2010.[9] The national and KZN statistics on average vitamin A supplementation as reported by DHIS are shown in Fig. 1.
Of the 923 RTHCs analysed, 647 had information recorded for both immunisation and vitamin A supplementation.
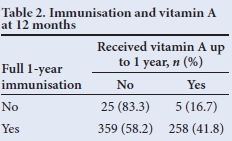
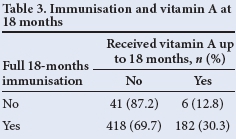
McNemar's χ2 test was used to test for association between receipt of full immunisation and vitamin A supplementation. It showed that receiving full immunisation for 12 months does not necessarily imply that full vitamin A supplementation is received - only 41.8% of those children who were fully immunised in the first 12 months of life received two doses of vitamin A (p<0.05) (Table 2). A similar pattern was seen at 18 months (Table 3), where only 30% of those who were fully immunised had received three vitamin A doses at 6-monthly intervals (p<0.05).
Discussion
Immunisation
The first year immunisation figure of 92.9% coverage is in keeping with the WHO target of coverage above >90%, and exceeds national figures as well as those of the province and many districts. The national immunisation coverage for 2006/2007 was 85.4% and for 2007/2008 was 84.2%.[3] This study population falls under the district of eThekwini and performed above the overall KZN provincial coverage of 78.8%,[3] according to DHIS data. Data from the current study were compared with national data from this timeframe (2007/2008) as it correlated to the time period when the youngest children in this study were >1 year of age, and was therefore comparable with DHIS data. Although data from this study exceeded national and district averages, it should be borne in mind that DHIS data are not without fault or human error, being dependent on clinic registers and population estimates, and subject to population migration. What is notable is the reported large variations between districts across the country, with some districts reporting coverage figures of 123% at 1 year of age, while others reported figures as low as 31%.[10]
What is unique about the data from this study is that it assessed the coverage for immunisations at 6 years of age (i.e. diptheria and tetanus (DT)1 and OPV5) and showed coverage of 48.3% for DT and 48.2% for OPV5. These data are not collected at a district or national level from registers and therefore no source data for comparison are available. One study in the Ga-Rankuwa community (Gauteng Province) found 20% coverage for DT1 and 40% for OPV5 for the year 2007.[2]
This study did not explain the difference in the DT1 and OPV5 coverage rates (as one would not expect them to differ), nor did it describe the decline in immunisation programme utilisation beyond the first year of life.
This IVAC study had a lifetime immunisation coverage (up to 5 years, in accordance with EPI definition) of 44%, the decline in which is probably attributable to underutilisation of healthcare facilities in later childhood, as children become more robust with stronger immune systems and less susceptible to life-threatening illness. Migration of communities and changes in primary caregivers may also be contributory.
The DPT1 - 3 immunisation dropout rate measures the percentage of children who dropped out between the first and the third dose of the DPT-Hib vaccine. This study detected a dropout rate for DPT1 - 3 of 1.2%, lower than the national 3.4% dropout rate for 2007/2008.[10] This shows good utilisation and access to immunisation schedules within the first year of life.
The measles dropout rate of 2.4% is below the overall dropout rate of >5% for the surrounding eThekwini area for 2006/2007. The poorest-performing province during this timeframe was the Free State, with a dropout rate of 14.7%.[10] However, some districts in the Western Cape performed better than the study population, with rates as low as 0.6% for the same timeframe.[10] There were no measles vaccination mass campaigns during this time to explain an improved immunisation coverage or improved dropout rate.
Vitamin A
The DHIS defines vitamin A coverage as two doses within a 1-year period between ages 12 and 59 months. As seen in Fig. 1, the coverage rate ranges from 14.4% in KZN and 12.8% nationally in 2004 to 32.8% in KZN and 34.6% nationally in 2010.[9]
DHIS data are collected by assessing registers from primary healthcare (PHC) facilities - the numerator is the number of vitamin A doses given to all children aged between 6 and 60 months and the denominator is the population estimate of children aged between 6 and 60 months, according to figures submitted to DHIS from Stats SA. RTHCs are not used in the assessment and the denominator is based on population estimates, so coverage statistics may not be completely accurate. In order to attempt to make the figures comparable we calculated the average coverage for this population (i.e. calculated actual number of doses given to all patients until 5 years of age, divided by the total number of doses they should have received in this timeframe).
The coverage of 34.9% (Fig. 1) across this population was similar to the coverage nationally and in KZN for years 2009 and 2010. We see a low level of coverage in the first few years of implementation, followed by a gradual increase and then a plateau from 2009, with an overall suboptimal uptake of the programme. This is surprising given the resources placed on education and promotion of the vitamin A supplementation programme.[11]
Tables 2 and 3 show that there was no correlation between those children who were fully immunised and vitamin A supplementation, indicating a weakness in our vitamin A supplementation programme, despite parents making use of healthcare facilities.
The 24 children who received no vitamin A doses had higher immunisation levels than the general population, indicating that the lack of recorded vitamin A doses was not an indicator of decreased use of the healthcare system.
Possible explanations for this discrepancy include the difference in timing and intervals between the vaccination schedule and vitamin A administration, as well as a possible lack of education and drive behind the vitamin A campaign.
The sobering findings of low coverage with vitamin A capsule supplementation in an area with a health system clearly capable of delivering vaccines suggests that there are factors other than health system capabilities at play. While this study is not able to unravel the causes for this low coverage, the data suggest a need for further exploration; we should go back to the evidence base and reassure ourselves that blanket vitamin A supplementation, in SA's current context, will save lives, and if this is true, urgently implement strategies to improve delivery. However, if, as has been suggested by several groups in the last few years, high-dose vitamin A supplementation to young children does not save lives to the extent that it was originally purported to, then we need a rethink.[12-14]
Mason et al.[12]point out that the studies that informed the current WHO guidelines were all conducted over 10 - 20 years ago in a world that was very different to the world of today: many more vaccines are available, diarrhoea and measles (the infections that vitamin A targeted) rates have diminished, and many foods are fortified with vitamin A.[13,14] The only study reported recently is a very large randomised control trial (RCT) conducted in India with over 1 million children, which showed no effect on mortality.[15] More concerning is a very recent report from Guinea-Bissau[16] of an RCT testing high-dose vitamin A or placebo given in conjunction with vaccines in children aged 6 - 23 months, which showed that vitamin A supplementation had no overall effect on mortality. Furthermore, in disaggregated data, boys tended to have an increase in mortality suggesting again the need for rethinking the current vitamin A capsule distribution policy.
Evidence is mounting for the following: vitamin A supplementation may no longer be a child survival strategy; and use of high pharmacological doses to correct any vitamin A deficiency may actually be dangerous. Therefore, food-based solutions seem more sensible and should be encouraged. In an impoverished community in the Northern Cape with poor anthropometric status (36.9% stunting), and where liver is routinely consumed by children and adults, the mean vitamin A intake in children 24 - 59 months of age was 537 μg.[17] This far exceeds the estimated average requirement for preschool children and suggests that food-based approaches are possible. It also suggests that caution needs to be taken in widespread roll-out of the IVAC distribution programme, as children who are vitamin A replete could be getting far more than they need.
Study limitations
Data were collected retrospectively and accuracy of documentation could not be assessed. Furthermore, coverage may be underestimated owing to doses not being accurately recorded, and therapeutic vitamin A doses given in hospital may not have been recorded. The data analysed were routinely collected and there is therefore a possibility of some inaccuracies in the data.
Owing to having children whose birth year spanned across 3 years and analysing the RTHCs in retrospect, this study was unable to break down the coverage for vitamin A in discrete timeframes for comparison with national data for one specific year. The initial RTHCs did not have the vitamin A data page copied, so vitamin A coverage was actually only assessed in the latter 70% of the cohort. There were however no special vitamin A mass campaigns or stock-outs during this study period, which would have lead to differential coverage.
This study did not look at possible socioeconomic reasons for vitamin A and immunisation success or failure. This would be beneficial, and is a suggested research avenue for further studies.
Conclusion
This study population showed good immunisation coverage compared with national and district data. Although the coverage of vitamin A supplementation was low, it was in line with national data, showing a relatively poor uptake of the vitamin A supplementation programme. There was no correlation between full immunisation status and high coverage of vitamin A supplementation, suggesting that good use of primary healthcare facilities for immunisation does not necessarily imply good uptake of the vitamin A supplementation programme. A failing vitamin A supplementation programme warrants a thorough evaluation as to the possible reasons for failure; this could be an opportune time to assess whether SA still needs an untargeted vitamin A capsule distribution programme. We believe the time has come to reconsider the value of blanket distribution of vitamin A capsules and rather consider targeted vitamin A for specific situations.
Acknowledgements. The authors acknowledge Prof. Meera Chhagan for providing access to the Asenze study data and for her contribution to the study design of this project. The authors would like to thank the staff of Asenze for their assistance as well as the participants of the Asenze study. We also acknowledge the local Health Committee and community health centre for their support.
References
1. World Health Organization (WHO). Review of National Immunization Coverage 1980 - 2006: South Africa. Geneva: WHO, 2007. [ Links ]
2. Wright S, Maja T, Furaha S. The impact of mothers' knowledge on the immunization of children younger than five in Ga-Rankuwa, South Africa. Afr J Nurs Midwifery 2011;13(2):29-42. [ Links ]
3. Day C, Barron P, Massyn N, Padarath A, English R. The District Health Barometer 2010/2011. Johannesburg: Health Systems Trust, 2011. [ Links ]
4. Black RE, Allen LH, Bhutta ZA, et al. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008;371(9608):243-260. [http://dx.doi.org/10.1016/S0140-6736(07)61690-0] [ Links ]
5. Mayo-Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: Systematic review and meta-analysis. BMJ 2011;343:d5094. [http://dx.doi.org/10.1136/bmj.d5094] [ Links ]
6. World Health Organization (WHO). Guideline: Vitamin A Supplementation in Infants and Children 6 - 59 months. Geneva: WHO, 2011. [ Links ]
7. Chhagan MK, Mellins AM, Kauchali S, et al. Mental health disorders among caregivers of preschool children in the Asenze Study in KwaZulu-Natal, South Africa. Matern Child Health J 2014;18(1):191-199. [http://dx.doi.org/10.1007/s10995-013-1254-5] [ Links ]
8. Uwemedimo O, Arpadi SM, Chhagan MK, et al. Compliance with referrals for non-acute child health conditions: Evidence from the longitudinal ASENZE study in KwaZulu-Natal, South Africa. BMC Health Serv Res 2014;14:242. [http://dx.doi.org/10.1186/1472-6963-14-2421 [ Links ]
9. National Department of Health. District Health Information System Database. http://indicators.hst.org.za/healthstats/241/data (accessed November 2014). [ Links ]
10. Ford-Ngomane T. Health Barometer Report 2007/2008. Output Indicators, Health Systems Trust. Johannesburg; Health Systems Trust, 2009. [ Links ]
11. Department of Health, South Africa. Roadmap for Nutrition in South Africa 2012 - 2016. www.doh.gov.za (accessed November 2013). [ Links ]
12. Mason J, Greiner T, Shrimpton R, Sanders D, Yukich J. Vitamin A policies need rethinking. Int J Epidemiol 2014;Oct:1-10, advance access publication. [http://dx.doi.org/10.1093/ije/dyu194] [ Links ]
13. Kapil U. Time to stop giving indiscriminate massive doses of synthetic vitamin A to Indian children. Public Health Nutr 2009;12(2):285-286. [http://dx.doi.org/10.1017/S1368980008004448] [ Links ]
14. Kapil U, Sachdev HP. Universal vitamin A supplementation programme in India: The need for a re-look. Natl Med J India 2010;23(5):257-260. [ Links ]
15. Awasthi S, Peto R, Read S, et al. Vitamin A supplementation every 6 months with retinol in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet 2013;381(9876):1469-1476. [http://dx.doi.org/10.1016/S0140-6736(12)62125-4] [ Links ]
16. Fisker AB, Bale C, Rodrigues A, et al. High-dose vitamin A with vaccination after 6 months of age: A randomized trial. Pediatrics 2014;134(3):e739-748. [http://dx.doi.org/10.1542/peds.2014-0550] [ Links ]
17. Nel J, van Stuijvenberg ME, Schoeman SE, Dhansay MA, du Plessis LM. Liver intake in 24 - 59 month-old children from an impoverished South African community provides enough vitamin A to meet requirements. Public Health Nutr 2014;17(12):2798-2805. [http://dx.doi.org/10.1017/S1368980013003212] [ Links ]
 Correspondence:
Correspondence:
V Comley
vanessacomley@yahoo.com














