Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Child Health
versión On-line ISSN 1999-7671
versión impresa ISSN 1994-3032
S. Afr. j. child health vol.9 no.2 Pretoria ene./abr. 2015
http://dx.doi.org/10.7196/SAJCH.901
RESEARCH
Hypoxaemia as a measure of disease severity in young hospitalised Nigerian children with pneumonia: A cross-sectional study
M B AbdulkadirI; R M IbraheemII; A A GobirIII; W B R JohnsonI
IMBBS, FWACP (Paed); Department of Paediatrics and Child Health, University of Ilorin and University of Ilorin Teaching Hospital, Ilorin, Nigeria
IIMBBS, FWACP (Paed), FMCPaed; Department of Paediatrics and Child Health, University of Ilorin and University of Ilorin Teaching Hospital, Ilorin, Nigeria
IIIMBBS, FMCPaed; Department of Paediatrics and Child Health, University of Ilorin and University of Ilorin Teaching Hospital, Ilorin, Nigeria
ABSTRACT
BACKGROUND: Pneumonia remains a common cause of mortality among children in developing countries. Hypoxaemia is a common consequence of pneumonia in children.
OBJECTIVES: To define the relationship between Hb oxygen saturation (SpO2) and parameters of outcome, duration of supplemental oxygen and duration of hospitalisation among children with pneumonia.
METHODS: A cross-sectional study was carried out at the paediatric wards of a tertiary hospital in North-Central Nigeria. Two hundred children aged between 2 and 59 months with pneumonia seen at the University of Ilorin Teaching Hospital were recruited consecutively. Sociodemographic and clinical information regarding the illness was obtained. Hb SpO2 of subjects was recorded with a pulse oximeter at presentation. The primary outcome was the SpO2 of the children with pneumonia. Secondary outcome measures were disease outcome, duration of supplemental oxygen and duration of hospitalisation among children with pneumonia.
RESULTS: The prevalence of hypoxaemia among the children was 41.5% and their mean SpO2 was 90.4% (standard deviation (SD) 8.9%). Surviving children with hypoxaemia had a longer mean (SD) duration of hospitalisation of 6.9 (6.4) days compared with those without hypoxaemia (4.9 (2.7) days; p=0.001). Children with hypoxaemia spent a longer duration receiving supplemental oxygen compared with those without hypoxaemia (p=0.001). The case fatality rate from pneumonia was 8.5% (17 deaths). The risk of death among children with hypoxaemia was 48 times higher than among the non-hypoxaemic children.
CONCLUSION: Hypoxaemia with increasing severity significantly predicts a longer duration of hospitalisation, duration on supplemental oxygen and poorer outcome in children with pneumonia.
Globally, pneumonia is a leading cause of death among children <5 years old, accounting for >90% of acute lower respiratory infection-related deaths. In Nigeria, pneumonia-related deaths account for 20 -25% of childhood mortality[1] A previous study from Ilorin in the North-Central region of Nigeria reported a case fatality rate of 10%.[2] Hypoxaemia is a major complication of pneumonia, associated with an increase in the risk of death with increasing severity of hypoxaemia.[3] It is often associated with acidosis, organ dysfunction and multiple complications.
Hypoxaemia can be detected via clinical signs, blood gas analysis or pulse oximetry. While blood gas analysis represents the 'gold standard' for defining hypoxaemia, its use is limited by its expense, invasiveness and provision of only a single measure per sample. However, pulse oximetry has been shown to be reliable, safe, non-invasive, simple and reproducible, hence most authors accept it as a detection tool.[3] Pulse oximetry has also been found to be superior to the use of clinical signs alone in detecting hypoxaemia.[4,5] It may be a useful tool in ensuring the most efficient use of oxygen therapy, which is especially important in resource-limited settings.
Despite the burden of mortality from pneumonia, there is a dearth of knowledge regarding the contribution of hypoxaemia to its outcome in the North-Central region of Nigeria. The objective of the current study therefore is to describe the relationship between various levels of Hb oxygen saturation (SpO2) and duration of hospitalisation, duration on oxygen and mortality, among a group of hospitalised children with pneumonia.
Methods
This was a descriptive cross-sectional study in which the subjects were children aged between 2 months and 5 years of age, who were diagnosed with pneumonia. The study was conducted at the Emergency Paediatric Unit (EPU) and the Paediatric Medical Ward (PMW) of the University of Ilorin Teaching Hospital (UITH). The hospital is a tertiary centre in Ilorin South Local Government Area of Kwara State in the North-Central geopolitical zone. The EPU and PMW cater for children from beyond the neonatal period to the age of 14 years. At the time of this study, the Hb SpO2 levels of children in the emergency room were not routinely measured, and therapeutic oxygen was commenced based on clinical assessment alone.
The sample size was calculated using the Fisher formula,[6,7] and a prevalence of pneumonia of 11.1% from a previous study.[2] The calculated minimum sample size was 151; however, a total of 200 children <5 years old were recruited for the study.
The subjects were children presenting at the EPU with clinical features comprising a cough of <28 days' duration, fever, difficult breathing, age-related tachypnoea (>50 breaths/minute for infants aged 2 months - 1 year, and >40 breaths/minute for children aged 12 - 59 months), and auscultatory findings of at least one of reduced breath sound intensity, bronchial breath sounds or crepitations.[4] All consecutive admissions into the EPU with a diagnosis of pneumonia were enrolled. The study was completed within 12 months (March 2010 - February 2011). Children with sickle cell disease, bronchial asthma, severe anaemia (haematocrit < 15%) and clinical features of shock, such as cold, clammy extremities, weak, thready pulse and other parameters of poor peripheral perfusion were excluded from the study.
The study was approved by the Ethics and Research Committee of the University of Ilorin Teaching Hospital. Written informed consent was obtained from all caregivers.
A semistructured questionnaire was administered to obtain the clinical and sociodemographic data from each subject's parent. Clinical observations were made and recorded. Hb SpO2 was measured by attaching a Smartsigns Liteplus CE 0088 pulse oximeter (Huntleigh Healthcare, UK) to a finger using an appropriately sized paediatric sensor. This was done as soon as possible after presentation, before oxygen administration if required. SpO2 was recorded after a stable reading was obtained for at least 1 minute, while the child was breathing room air. For the purpose of the study, hypoxaemia was defined as an SpO2 of <90% as recorded by pulse oximetry.[5] In addition, the various levels of SpO2 were categorised into five groups: >95%, 93 - 95%, 90 - 92%, 86 - 89%, and <85%.
The severity of pneumonia in each subject was graded as mild, moderate or severe using the British Thoracic Society guidelines on the management of community-acquired pneumonia in children.[8] Subjects with complications of pneumonia at presentation were considered as having severe pneumonia.[8] Chest radiographs were obtained for all subjects within 24 hours of presentation. Radiographic features were recorded as either normal, presence of patchy opacities in one or more lobes, or lobar/segmental consolidation with or without an air bronchogram. The radiograph findings were corroborated by a consultant radiologist.
All subjects were treated with the most appropriate medications according to the current institutional guidelines. Each child was followed up to monitor the admission outcome (survived or died). The duration on supplemental oxygen (for those given) and hospitalisation were also documented.
Statistical analyses
Data were analysed using SPSS version 20.0 (IBM, USA) for Windows. The data collected on the proforma were transferred onto a master sheet using numerical codes. After the generation of frequency tables and simple proportions, the χ2 and Student's t-tests were used to identify significant differences for categorical and continuous variables, respectively. Case fatality rates were calculated for the various cut-offs of SpO2. Relative risk of death among the hypoxaemic children was calculated. A p-value of <0.05 was considered significant.
Results
Two hundred patients were recruited, and all patients completed the study. The mean (SD) age of the children with pneumonia was 14.3 (13.5) months. A total of 113 (56.5%) children were aged <12 months, 46 (23.0%) were aged 12 - <24 months, 26 (13.0%) were aged 24 - <36 months, 4 (2.0%) were 36 - <48 months, and 11 (5.5%) were 48 - <60 months. Overall, 119 (59.5%) patients were male.
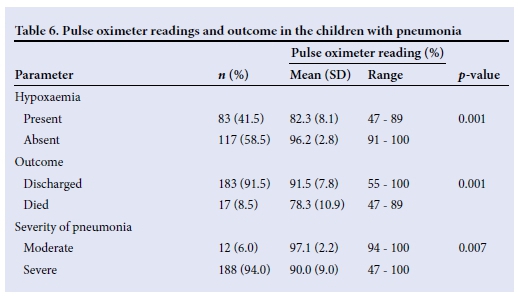
Using the defined cut-off for hypoxaemia, namely SpO2 <90%, 83 children (41.5%) had hypoxaemia (Table 1). The mean (SD, range) SpO2 was 90.4% (8.9, 47 - 100), while mean (SD) SpO2 values among the hypoxaemic and non-hypoxaemic children were 82.3% (8.1) and 96.2% (2.8), respectively.
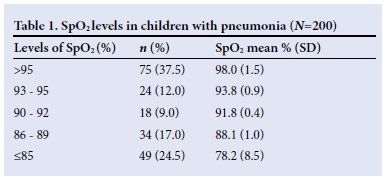
The mean duration of hospital admission among the subjects recruited was 5.7 (4.7) days. Table 2 shows that the children who had hypoxaemia had a significantly longer duration of hospitalisation compared with those without hypoxaemia (p=0.002).
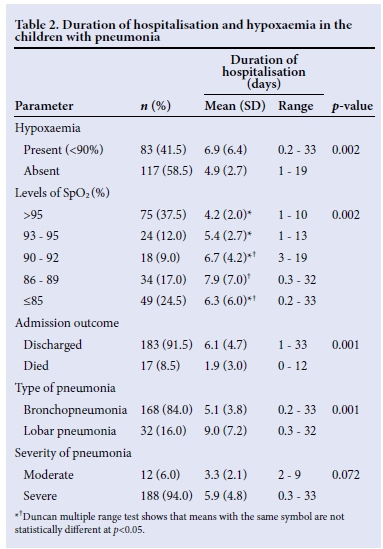
The mean duration of hospitalisation increased as SpO2 levels decreased. Furthermore, the mean duration of hospitalisation in children with lobar pneumonia was significantly longer than the corresponding values for those with bronchopneumonia (p=0.001). Among the survivors, the children with hypoxaemia had a longer duration of hospitalisation compared with those without hypoxaemia (p=0.001) (Table 3).
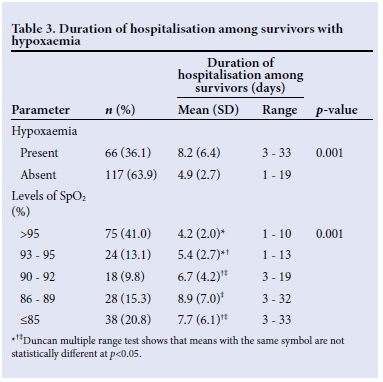
The mean (SD) duration of supplemental oxygen therapy among all the subjects was 26.3 (34.5) hours. The mean duration on supplemental oxygen to the children with hypoxaemia was significantly longer than the corresponding value recorded in those without hypoxaemia (p=0.001) (Table 4). The mean duration of oxygen therapy in children with pneumonia increased significantly as the SpO2 levels decreased (p=0.001).
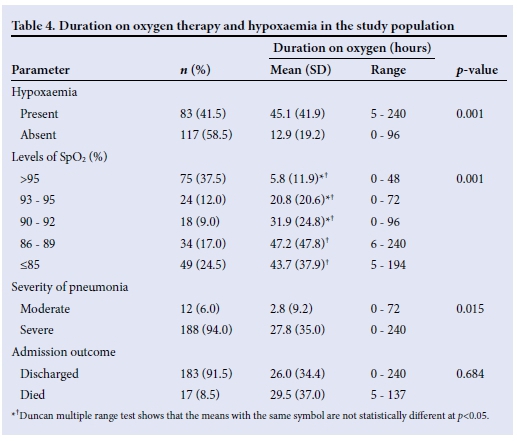
Seventeen of the children with pneumonia died, giving a case fatality of 8.5%. Of these, 10 (58.8%) were aged <12 months, 3 (17.7%) were aged 12 - <24 months, and 4 (23.5%) were aged 24 - <36 months. Twelve (70.6%) of the 17 children who died were male. All the children who died had hypoxaemia (Table 5). The case fatality rate for children with hypoxaemia was 20.5% with a relative risk of death of 48 in children with hypoxaemia compared with those without. Regarding the various cut-offs for hypoxaemia, there was a progressive increase in case fatality rate as SpO2 fell to <90% (Table 5).
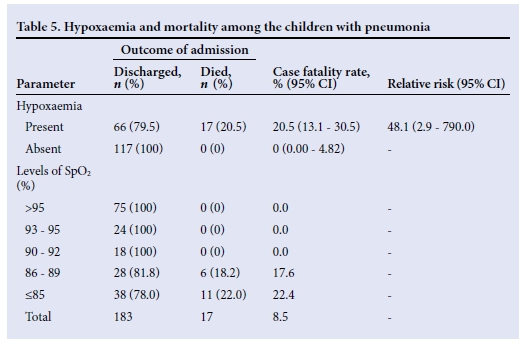
The mean SpO2 level recorded of 78.3% (10.9) among the fatal cases was significantly lower than the corresponding value of 91.5% (7.8) recorded among the children who survived (p=0.001). The relationship between the recorded pulse oximeter values and various other parameters is shown in Table 6.
Discussion
The case fatality among the children with pneumonia in this series was 8.5%. While this value is slightly higher than the 7.8% recorded by Johnson et al.[9] in Ibadan, an even higher case fatality value of 10.0% had been identified in an earlier report (some 25 years earlier) by Fagbule et al.[2] in Ilorin, where the present study was carried out. The corresponding values from other countries include 15.0% reported by Nathoo et al.[10] in Zimbabwe and 10.5% by Sehgal et al.[11]in India. It is worrisome that despite advances in pneumonia case management, including appropriate use of antibiotics and deployment of technology, there has been only a paltry 15% decrease in case fatality over the past 25 years. Some of this barely significant decline may be attributed to more prompt home recognition of disease severity, early diagnosis, better defined criteria for referrals, as well as institutional adoption of more effective management strategies in the last few years.[11,12] However, there still exists an urgent need to improve pneumonia case management and its outcome drastically.
The fact that the presence of hypoxaemia was associated with a significantly higher pneumonia-related mortality is in accord with earlier reports.[13] Pneumonia interferes with the process of oxygen exchange at the alveoli and increases ventilation-perfusion mismatch. Thus, it is conceivable that more-severe disease will further limit oxygen exchange, leading to hypoxaemia. This emphasises the role of SpO2 as a tool for determining initial disease severity. Similar reports have shown the presence of hypoxaemia to correlate with severity of pneumonia.[12] This study further validates the definition of hypoxaemia as SpO2 <90%, as case fatality rate was nil in children with SpO2 >90%, but progressively increased with lower SpO2 levels. The presence of hypoxaemia increased the risk of death 48-fold compared with those who were non-hypoxaemic. The preponderance of children with severe disease in this study as shown by a prevalence of hypoxaemia of 42% compared with 5.8% in The Gambia[14] and 6.4% in Kenya[13] provides some explanation for the wide disparity in the hypoxaemia-related risk of death. Other reasons may relate to the smaller sample size in this study and the possible differing levels of care provided in these facilities. Nevertheless, the implication of this dramatic increase in risk of death is the need to evolve systems for aggressive management of those patients presenting with hypoxaemia in developing countries.
In the current study, the duration of hospital stay was found to be significantly longer for hypoxaemic children. This observation is similar to the reported findings in some earlier studies.[13,14] Indeed, the mean duration of hospitalisation increased as the levels of hypoxaemia worsened, with decreasing SpO2 levels. This is attributable to the longer time required by hypoxaemic children with pneumonia to recover from the underlying pathophysiological aberrations of alveolar hypoventilation and ventilation-perfusion mismatch.
Supplemental oxygen is given to children with pneumonia to relieve hypoxaemia. In the current study, the mean duration of supplemental oxygen administration increased with decreasing SpO2 and severity of pneumonia. This has strong implications in developing countries where oxygen may be scarce. An identified limitation of the study was the use of single-point determination of SpO2 rather than a continuous measure, which would have provided a more accurate guide to the actual duration for which a patient requires supplemental oxygen. Nevertheless, in situations where oxygen supplies are limited and facilities for monitoring saturation are not available, initial disease severity may be a reliable guide to planning the rationing of such supplies. These data underscore the need to make pulse oximeters available in healthcare facilities with the capacity and wherewithal for administering oxygen therapy to patients.
Conclusion
The prevalence of hypoxaemia in children <5 years of age hospitalised with pneumonia is 41.5%, and hypoxaemia significantly predicts a worse outcome in terms of mortality, duration of hospitalisation and oxygen therapy. Thus, it would be essential for health facilities in developing countries to have capacity for monitoring SpO2 as a guide to oxygen therapy and aggressive management.
Acknowledgements. The authors acknowledge the contributions of all the consultants, residents and entire nursing staff of the EPU, and thank the parents who consented to be part of this study.
References
1. Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bull World Health Organ 2008;86(5):408-416. [ Links ]
2. Fagbule D, Adedoyin M, Nzeh D. Childhood pneumonia in the University of Ilorin Teaching Hospital. Nig J Paediatr 1987;14(3,4):73-78. [ Links ]
3. Mower WR, Sachs C, Nicklin EL, Baraff LJ. Pulse oximetry as a fifth pediatric vital sign. Pediatrics 1997;99(5):681-686. [ Links ]
4. Stein RT, Marostica PJC. Community-acquired pneumonia: A review and recent advances. Pediatr Pulmonol 2007;42(12):1095-1103. [http://dx.doi.org/10.1002/ppul.20652] [ Links ]
5. Hassan A. Non-invasive monitoring of blood gases. In: Hassan A, ed. Handbook of Blood Gas / Acid Base Interpretation. London: Springer-Verlag; 2009:63-95. [ Links ]
6. Araoye MO. Subjects selection. In: Araoye MO, ed. Research Methodology with Statistics for Health and Social Sciences. Ilorin: Nathadex, 2003:115-121. [ Links ]
7. Fisher AA, Laing JE, Stoeckel JE, Townsend JW. Handbook for Family Planning Operations Research Design. 2nd ed. New York: Population Council, 1991:43-46. [ Links ]
8. Harris M, Clark J, Coote N, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: Update 2011. Thorax 2011;66(Suppl 2):ii1-23. [http://dx.doi.org/10.1136/thoraxjnl-2011-200598] [ Links ]
9. Johnson WBR, Osinusi K, Aderele WI, Gbadero D. Acute lower respiratory infections in hospitalized urban preschool Nigerian children: A clinical overview. Afr J Med Med Sci 1994;23(2):127-138. [ Links ]
10. Nathoo KJ, Nkrumah FK, Ndlovu D, Nhembe M, Pirie DJ, Kowo H. Acute lower respiratory tract infection in hospitalized children in Zimbabwe. Ann Trop Paediatr 1993;13(3):253-261. [ Links ]
11. Sehgal V, Sethi GR, Sachdev HP, Satyanarayana L. Predictors of mortality in subjects hospitalized with acute lower respiratory tract infections. Indian Pediatr 1997;34(3):213-219. [ Links ]
12. Principi N, Esposito S. Management of severe community-acquired pneumonia of children in developing and developed countries. Thorax 2011;66(9):815-822. [http://dx.doi.org/10.1136/thx.2010.142604] [ Links ]
13. Onyango FE, Steinhoff MC, Wafula EM, Wariua S, Musia J, Kitonyi J. Hypoxaemia in young Kenyan children with acute lower respiratory infection. BMJ 1993;306(6878):612-615. [ Links ]
14. Usen S, Weber M, Mulholland K, et al. Clinical predictors of hypoxaemia in Gambian children with acute lower respiratory tract infection: Prospective cohort study. BMJ 1999;318(7176):86-91. [ Links ]
 Correspondence:
Correspondence:
M B Abdulkadir
docmohng@gmail.com














