Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.9 n.2 Pretoria Jan./Apr. 2015
http://dx.doi.org/10.7196/SAJCH.803
RESEARCH
Determinants of mother-to-child transmission of HIV despite PMTCT interventions in Enugu, Nigeria
K K IlohI; O N IlohI; A N IkefunaI; N S IbeziakoI; A C UbesieII; I J EmodiI
IMBBS; Department of Paediatrics, University of Nigeria Teaching Hospital, Ituku/Ozalla, Enugu, Nigeria
IIMBBS, MPH; Department of Paediatrics, University of Nigeria Teaching Hospital, Ituku/Ozalla, Enugu, Nigeria
ABSTRACT
BACKGROUND: The burden of paediatric HIV is unacceptably high in Nigeria. Prevention of mother-to-child transmission (PMTCT) of HIV represents a critical opportunity for reducing the burden of paediatric HIV.
OBJECTIVES:To determine risk factors of MTCT of HIV following PMTCT interventions.
METHODS: This was a prospective study over a 12-month period, involving HIV-positive pregnant mothers in their third trimester. A structured, interviewer-administered questionnaire was used to obtain relevant information about mothers and their babies. Maternal HIV RNA levels (viral load) and CD4 counts were also obtained. DNA polymerase chain reaction (PCR) testing was done for all the infants. Data analysis was with SPSS version 15 (Chicago, USA).
RESULTS: There was a total of 210 infants, comprising 198 singletons and 6 sets of twins. Two infants had a positive DNA PCR, giving an MTCT rate of 1%. There was significant association between MTCT of HIV and maternal HIV RNA levels (p=0 .009) and mixed feeding (p<0.001). None of the other risk factors studied, namely maternal CD4 count, mode of delivery and duration of rupture of fetal membrane before delivery, had any influence on MTCT.
CONCLUSION: The rate of MTCT can be reduced markedly if there is strict adherence to PMTCT strategies. It is therefore recommended that there be increased access to PMTCT programmes and full participation of mothers in Nigeria.
Worldwide, an estimated two million infants of HIV-infected pregnant mothers are exposed to HIV annually.[1] Two hundred and fifty thousand children died of AIDS-related illnesses in 2010 alone.[1] The national seroprevalence rate of HIV in Nigeria stood at 4.1% in 2010.[2]
More than 90% of paediatric HIV infections occur through mother-to-child transmission (MTCT).[3] Fifteen to forty per cent of infants born to HIV-infected mothers become infected in utero, during labour and delivery, or by breastfeeding postnatally.[4] For non-breastfeeding populations, 50% of HIV infections are transmitted to infants towards the end of pregnancy, during labour and delivery, while for breastfeeding populations, the postnatal period accounts for most of the HIV infections transmitted to infants.[5]
Estimated rates of MTCT among untreated, seropositive women vary between 15 and 25% in Europe, to 25 and 40% in Africa and Asia.[6] In Nigeria, the estimated transmission rate among untreated mothers is reported to be as high as 45%.[7] Transmission rates of HIV are consistently higher in resource-poor countries than in industrialised countries.[6] Transmission rates <2% have been documented in developed countries.[8,9] Studies in Nigeria have reported transmission rates of 4 - 16% following the use of antiretroviral (ARV) drugs and safe obstetric practices.[10-12]
The implementation of the PMTCT programme in Nigeria commenced in July 2002.[13] By 2008, there were 640 sites across the country where PMTCT had been integrated into routine antenatal care services. Interventions for the PMTCT of HIV in University of Nigeria Teaching Hospital (UNTH), Enugu, started in 2006 and include the use of ARV drugs for the mother and her baby, safe delivery practices and appropriate infant feeding options. There is a need to evaluate the determinants of MTCT of HIV in Enugu, which is critical for the monitoring, evaluation and updating of preventive strategies.
Methods
Study area and site
This study was carried out at UNTH, Ituku-Ozalla, in Enugu State, Nigeria. The study site was the HIV clinic, comprising a paediatric, an adult and a PMTCT clinic. The clinics have been improved with funding from President's Emergency Plan for AIDS Relief (PEPFAR). The PMTCT clinic is made up of two units:
• The antenatal unit, where infected pregnant women are seen from booking. The pregnant women are followed up until delivery, after which the baby is referred to the exposed babies clinic.
• The exposed babies clinic is where the babies born to HIV-infected women are followed up until their definite status is known. The HIV status is determined after 6 weeks of age using DNA polymerase chain reaction (PCR). For the infants who are being breastfed, the DNA PCR is repeated 6 weeks after breastfeeding has been stopped. All HIV-infected babies are referred to the ARV therapy (ART) clinic. HIV-negative babies remain with the follow-up clinic until 18 months of age, when a final ELISA test is done.
Study population
The subjects were HIV-infected pregnant women receiving antenatal care at UNTH, and their infants seen at the PMTCT follow-up clinic in UNTH. PEPFAR supports a full range of HIV services in the hospital: adult, paediatric and PMTCT. Therefore, it is not uncommon for women of reproductive age on treatment for their own health to be linked to the PMTCT services once they become pregnant.
HIV-positive pregnant women in their last trimester and their delivered infants seen within 72 hours of delivery were included in the study. Details of the study were explained to each HIV-positive mother by the researcher, and written informed consent was obtained before enrollment into the study. The exclusion criterion was eligible mothers who declined consent.
Study design
In this prospective cohort study, HIV-infected women who came to the PMTCT clinic who met the inclusion criteria were recruited consecutively on weekly clinic days until the desired sample size was achieved.
Ethical considerations
Ethical approval was obtained from the Health Research and Ethics Committee of UNTH. The names of all the study subjects were coded in the final soft and hard copies of the proforma to ensure confidentiality. All the relevant data pertaining to the study are in the sole custody of the principal investigator.
Data collection
Gestational age was determined using the mother's last menstrual period and ultrasound reports. Mothers were interviewed to obtain their sociodemographic profile: age, sex, parity, level of education, occupation, marital status and place of residence. The World Health Organization (WHO) HIV clinical stage and maternal ART use were also documented. CD4 count and viral load (VL) were requested. Infant feeding counselling messages were reinforced. The mothers were assigned a social class using Oyedeji's[14] social classification scheme (Appendix 1).
Each enrolled mother was seen with her baby within 72 hours of delivery, either at the labour room, in the postnatal ward or at the exposed babies clinic. Information on the gestational age at delivery, mode of delivery, duration of rupture of membrane, use of episiotomy and delivery instruments was extracted from the delivery form. Each mother was interviewed again to confirm her infant feeding choice and encouraged to adhere to her choice. The babies were examined and ART prophylaxis was commenced (nevirapine 2 mg/kg stat and zidovudine 4 mg/kg/dose every 12 hours for 6 weeks, according to the National Guidelines[15] (in use at the time of this study). At 6 weeks of age, irrespective of the feeding choice, a dry blood spot specimen was collected from each baby on a Whartman filter paper for HIV status determination using the DNA PCR technique. Pneumocystis pneumonia prophylaxis with co-trimoxazole (at a dose of 6 - 8 mg/ kg/day of trimethoprim component) was commenced and zidovudine stopped. At another scheduled visit, the result of the DNA PCR was discussed with the mother and her partner (if present). HIV-positive babies were referred to the paediatric ART clinic for evaluation and commencement of highly active ART (HAART). Those with negative DNA PCR and on exclusive replacement feeding with infant formula were encouraged to continue their routine follow-ups at the clinic. For babies with negative DNA PCR who were on exclusive breastfeeding, the test was repeated at least 6 weeks after breastfeeding had been stopped.
Early infant diagnosis
The PCR test, Ampliclor 3.2.1 (Roche Molecular Systems, USA) (100% sensitivity after 4 - 6 weeks of delivery[16]) was used for early infant diagnosis. Positive results were repeated on the same sample for confirmation.
Data analysis
Data were analysed using SPSS version 15.0 (IBM, USA). Descriptive statistics were used to summarise quantitative variables (age, weight), while qualitative variables (occupation, marital status, baby's sex, mode of delivery, DNA PCR status) were summarised by proportions. Student's t-test, χ2 and Fisher's exact test were used to test for significance between variables. A p-value <0.05 was considered statistically significant. All reported p-values were two-sided.
Results
Sociodemographic data
A total of 204 of HIV-positive mothers met the inclusion criteria and were recruited and studied over a 12-month period from February 2011 to January 2012. None of them refused consent. The mean age of mothers was 32 (standard deviation (SD) 4.05) years. Ninety-one (44.6%) had secondary education as their highest educational attainment, while 39 (19.1%) and 73 (35.8%) had attained primary and tertiary education, respectively. One (0.5%) had no formal education. The predominant socioeconomic class was social class III (Appendix 1), which accounted for 49.5% of the mothers.
Obstetric characteristics of mothers
The parity of 155 (76%) of the mothers was 2 - 4. A total of 196 (96.1%) of the pregnancies were of term gestation, while 8 (3.9%) were preterm. Overall, 173 (84.8%) mothers delivered vaginally, while 14 (6.9%) and 17 (8.3%) delivered by elective and emergency caesarean section, respectively. Of the 190 mothers who delivered by either par vaginam or through emergency caesarean section, 167 (88.8%) had their fetal membrane ruptured for <4 hours before delivery.
Overall, 197 (96.6%) mothers knew their status before the index pregnancy. Most of the mothers (n=143, 70.1%) were in WHO clinical stage 2. A total of 175 (85.8%) were on HAART before the index pregnancy, while 29 (14.2%) had ARV prophylaxis initiated within the pregnancy period. The CD4 count was >200 cells/mL in 192 (94.1%) of the mothers, and <200 in 12 (5.9%). HIV RNA levels were <400 viral copies/mL in 141 (69.1%) of the mothers, between 400 and 9 999 viral copies/mL in 36 (17.6%) and >10 000 viral copies/mL in 27 (13.2%).
Characteristics of the HIV-exposed infants
There were 210 babies delivered to the 204 HIV-infected mothers, comprising 198 singletons and 6 sets of twins. Of the 210 babies, 100 (47.6%) were males and 110 (52.4%) were females, giving a male:female ratio of 1:1.1. A total of 202 babies (96.2%) were of term gestation. The mean (SD) birth weight of the babies was 3.02 (0.5) kg. All the babies received postnatal ARV prophylaxis.
One hundred and thirty-seven infants (65.2%) went exclusively onto infant formula, 71 (33.8%) were exclusively breastfed, and only 2 (1%) had mixed feeding (breastmilk and formula). Sixty-four (90.1%) of the breastfed babies were exclusively breastfed for 3 months, while 2 (2.8%) and 5 (7.1%) were breastfed exclusively for 2 months and 1 month, respectively (Table 1).
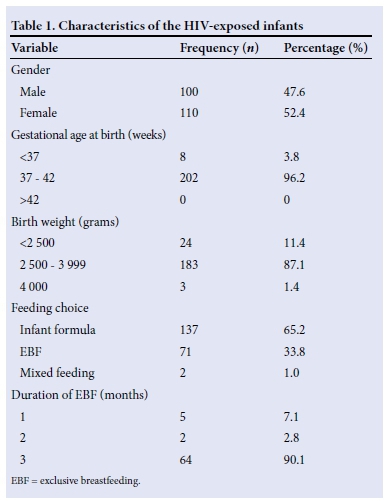
All the babies studied had their initial DNA PCR within 6 -8 weeks of delivery, and those who were breastfed had a repeat DNA PCR within 6 - 8 weeks after cessation of breastfeeding. Two hundred and eight (99%) were HIV-negative, while 2 (1%) were HIV-positive, giving a transmission rate of 1%.
Risk factors of MTCT of HIV
Maternal HIV RNA VL levels
Of the 145 infants whose mothers' VL was suppressed (<400 viral copies/mL) and 36 infants whose mothers VL was between 400 and 9 999 viral copies/mL, none had positive DNA PCR, whereas 2 of the 29 infants whose mothers' VL was >10 000 viral copies/mL had positive DNA PCR. The association between infants' DNA PCR results and high maternal HIV RNA levels (>10 000 viral copies) was statistically significant (p=0.009).
CD4 count
Whereas 2 of the 197 babies whose maternal CD4 count was > 200 cells/mL had positive DNA PCR, none of the 13 infants whose maternal CD4 count was <200 cells/mL had positive DNA PCR; however, the difference was not statistically significant (p=1.000).
Mode of delivery
Two of the 177 of the infants delivered vaginally had positive DNA PCR, whereas none of the infants delivered by elective or emergency caesarean section had positive DNA PCR; however, the difference was not statistically significant (p=1.000).
Feeding choice
HIV-positive babies were found only among those who received mixed feeding. There were none who were either exclusively breastfed or exclusively formula fed. The relationship between feeding choice and DNA PCR result was statistically significant (p<0.001).
Discussion
The MTCT rate of 1% in this study was lower than rates reported in previous studies in Nigeria.[7,17,18] A previous study in Nigeria among ARV-naive, HIV-infected pregnant women who had no preventive intervention reported an HIV MTCT rate of 45%.[7] The rates reported in other Nigerian studies where some form of PMTCT interventions were provided varied from 4 to 8%.[17,18] This could be owing to the fact that most of the women in this study knew their status before pregnancy, and had been on HAART with their viral RNA levels significantly suppressed. Additionally, the infants were started on ARV prophylaxis and most of the mothers adhered to the chosen infant feeding choice.
None of the women with a VL <9 999 viral copies/mL transmitted the virus to their infants. There have been consistent reports that maternal viral suppression has been associated with a significant reduction in the risk of MTCT of HIV.[8,18] In a multicentre, French perinatal cohort study, Warszawski et al.[8]documented a transmission rate of 0.6% in women whose VL was suppressed <400 viral copies/mL. The importance of marked maternal viral suppression was also documented by Garcia et al.[19]in the USA. Interestingly, the current study documented a transmission rate of 6.9% (2/29) among infants whose mother's VL was >10 000 viral copies/mL. This situation involved only a few mothers, but contributed to 100% of the infected children. Therefore, maternal VL clearly stands out as a key determinant of MTCT risk.
Although low CD4 count (<200 cells/mL) has been associated with increased risk of vertical transmission,[20] this was not the finding in this study, as the two mothers who transmitted the virus to their infants had CD4 counts of > 200 cells/mL. The explanation for this is not clear, but may be owing to the small proportion of the subjects studied who had a CD4 count <200 (5.9%) cells/mL.
Babies delivered vaginally in this study had a higher rate of transmission than those delivered by elective caesarean section. This finding is in consonance with previous studies that documented increased transmission in babies delivered by the vaginal route compared with those delivered by elective caesarean section.[21] The present study documented that none of the women whose VL was suppressed (<400 copies/mL) and who delivered per vaginam transmitted the virus to their infants.
A striking observation from this study was that there was no HIV-positive result among the infants who were exclusively breastfed. The use of HAART among most of our mothers and attendant VL suppression explains this. This finding is similar in a Ugandan study where Homsy et al.[22]observed that none of 114 infants who were exclusively breastfed tested positive for HIV. Other studies have also documented the importance of ART with resultant significant viral suppression in the reduction of risk of MTCT of HIV among infants who were exclusively breastfed.[17,23]
Also in this study, no transmission of HIV was found among infants who had exclusive replacement feeding, which agreed with the study in Jos University Teaching Hospital by Achonga.[11] The only two HIV-positive babies found in the current study had mixed feeding. Several studies have documented increased risk of MTCT of HIV among infants who were mixed fed.[17,24,25]
It is noteworthy that the transmission rate in this study is comparable with a rate of <2% such as documented in developed countries.[8-10] The results of this study clearly suggest a positive affect of the PMTCT strategies adopted in Nigeria and highlight the fact that the goal of virtual elimination, where no HIV-exposed child becomes infected, is possible even in resource-poor settings.
Conclusions
The rate of MTCT of HIV in UNTH, Enugu, among those who fully participated in the PMTCT programme was 1%. Significant risk factors of MTCT of HIV in this study were maternal HIV RNA levels and infant feeding choice.
Acknowledgement. We are thankful to the mothers and their infants who participated in this study.
References
1. UNAIDS. Global report: UNAIDS report on the global AIDS epidemic 2010. http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf (accessed 16 January 2014). [ Links ]
2. Federal Ministry of Health Nigeria. A Technical Report on 2010 National HIV sero-prevalence sentinel survey. Abuja: Federal Ministry of Health, 2010. [ Links ]
3. Car LT, van Velthoven MHMMT, Brusamento S, et al. Integrating prevention of mother-to-child HIV transmission programs to improve uptake: A systematic review. PLoS ONE 2012;7(4):e35268. [http://dx.doi.org/10.1371/journaLpone.0035268] [ Links ]
4. Lu D, Liu J, Samson L, et al. Factors responsible for mother-to-child HIV transmission in Ontario, Canada, 1996-2008. Can J Public Health 2014;105(1):e15-e20. [ Links ]
5. Kourtis AP, Lee FK, Abrams EJ, Jamieson DJ, Bulterys M. Mother-to-child transmission of HIV: Timing and implications for prevention. Lancet Infect Dis 2006;6(11):726-732. [http://dx.doi.org/10.1016/S1473-3099(06)70629-6] [ Links ]
6. Working Group on Mother-to-Child Transmission of HIV. Rate of mother-to-child transmission of HIV in Africa, America and Europe: Result of 13 perinatal studies. J Acquir Immune Defic Syndr 1995;8(5):506-510. [ Links ]
7. Odaibo GN, Olaleye DO, Heyndrickx L, Vereecken K, Houwer K, Jansens N. Mother-to-child transmission of different HIV subtypes among ARV-naïve infected pregnant women in Nigeria. Rev Inst Med Trop Sao Paulo 2006;48(2):77-80. [http://dx.doi.org/10.1590/S0036-46652006000200004] [ Links ]
8. Warszawski J, Tubiana R, le Chenadec J, et al. Mother-to-child HIV transmission despite anti-retroviral therapy in ANRS French Perinatal Cohort. AIDS 2008;22(2):289-299. [http://dx.doi.org/10.1097/QAD.0b013e3282f3d63c] [ Links ]
9. Ammann AJ. Human immunodeficiency virus in China: An opportunity to halt an emergency epidemic. AIDS patient care STD 2000;14(1):109-112. [ Links ]
10. Audu RA, Salu OB, Musa AZ, et al. Estimation of the rate of mother-to-child transmission in Nigeria. Afr J Med Sci 2006;35(2):121-124. [ Links ]
11. Achonga M. Infants of HIV-positive mothers at Jos University Teaching Hospital: Pattern of feeding and health status during the first six months of life (Thesis). Jos, Nigeria: National Postgraduate College Nigeria, 2006:33. [ Links ]
12. Ibeziako NS, Ubesie AC, Emodi IJ, Ayuk AC, Iloh KK, Ikefuna AN. Mother-to-child transmission of HIV: The pre-rapid advice experience of UNTH Ituku/Ozalla, Enugu, South-east Nigeria. BMC Res Notes 2012;5:305. [http://dx.doi.org/10.1186/1756-0500-5-305] [ Links ]
13. Federal Ministry of Health. Evaluation of the prevention of mother-to-child transmission (PMTCT) of HIV pilot programmme in Nigeria. Abuja: Federal Ministry of Health Nigeria, 2005:12. [ Links ]
14. Oyedeji GA. Socio-economic and cultural background of hospitalized children in Ilesha. Nig J Paed 1985;12(4):111-117. [ Links ]
15. Federal Ministry of Health. National Guideline for Paediatric HIV and AIDS Treatment and Care. Abuja: Federal Ministry of Health Nigeria, 2007. [ Links ]
16. Quiagen. Quiagen Genomic DNA Handbook. United States: Pharmacia Biotec, 2001:7. http://www.qiagen.com/ng/resources/resourcedetail?id=97640bc9-e4fe-4c4b-83f6-ac7ca4181597&lang=en (accessed 16 January 2014). [ Links ]
17. Okechukwu AA, Abdulrahaman IB. The impact of prevention of mother-to-child transmission of HIV programme in Federal Capital Territory, Abuja. Nig J Med 2008;17(2):191-197. [ Links ]
18. Sadoh WE, Sadoh AE, Adeniran KA, et al. Infant-feeding practices among HIV-infected mothers in an HIV treatment programmme. J Health Popul Nutr 2008;4:463-467. [ Links ]
19. Garcia PM, Kalish LA, Pitt J, et al. Maternal levels of plasma HIV type-1 RNA and the risk of perinatal transmission. N Engl J Med 1999;341(6):394-402. [ Links ]
20. The International Perinatal HIV Group. Duration of rupture of membrane and vertical transmission of HIV: A meta-analysis from 15 prospective cohort studies. AIDS 2001;15(3):357-368. [ Links ]
21. Thorne C, Semenenko I, Pilipenko T, Malyuta R. Progress in prevention of mother- to-child transmission of HIV infection in Ukraine: Results from a birth cohort study. BMC Infect Dis 2009;9:40. [http://dx.doi.org/10.1186/1471-2334-9-40] [ Links ]
22. Homsy J, Moore D, Barasa A. Breastfeeding, mother-to-child HIV transmission, and mortality among infants born to HIV-infected women on highly active antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr 2010;53(1):28-35. http://dx.doi.org/10.1097/QAI.0b013e3181bdf65a] [ Links ]
23. Palombi L, Marazzi M, Veotberg A, Magid NA. Treatment acceleration programme and the experience of the DREAM programme in the prevention of mother-to-child transmission of HIV. AIDS 2007;21(Suppl 4):S65-71. [http://dx.doi.org/10.1097/01.aids.0000279708.09180.f5] [ Links ]
24. Piwoz EG, Humphery JM, Tavengwa NV, et al. The impact of safer breastfeeding practices on postnatal HIV transmission in Zimbabwe. Am J Pub Health 2007:97(7):1249-1254. [http://dx.doi.org/10.2105/AJPH.2006.085704] [ Links ]
25. Coovadia HM, Rollins NC, Bland RM, et al. Mother-to-child transmission of HIV infection during exclusive breastfeeding in the first six months of life: An interventional cohort study. Lancet 2007;369(9567):1107-1116. [http://dx.doi.org/10.1016/S0140-6736(07)60283-9] [ Links ]
 Correspondence:
Correspondence:
A C Ubesie
zionagoz@yahoo.co.uk
Appendix 1. Oyedeji's socioeconomic index scores
Educational attainments:
- Class I: University graduates or equivalents
- Class II: School certificate holders with teaching or other professional training
- Class III: School certificate or Grade 2 teachers' certificate holders or equivalents
- Class IV: Modern 3 or primary six certificate holders
- Class V: Cannot read/write or illiterate
Occupation of parents:
- Class I: Senior public servants, professionals, managers, large-scale traders, businesspeople, contractors
- Class II: Intermediate group of public servants and senior school teachers
- Class III: Junior public servants and school teachers, drivers, artisans
- Class IV: Petty traders, labourers and similar grades
- Class V: Unemployed, full-time housewives, students and subsistence farmers Calculation of socioeconomic class (SEC)
Four scores were obtained from educational attainment and occupation for the two parents. The socioeconomic grading is obtained by finding the mean of the four scores for the two parents. This is then rounded off to the nearest whole number to get the SEC level of the study participant.














