Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.9 n.2 Pretoria Jan./Apr. 2015
http://dx.doi.org/10.7196/SAJCH.755
RESEARCH
The Micral-Test as a screening tool to detect microalbuminuria in children 5 - 15 years old with sickle cell anaemia, Lagos State University Teaching Hospital
A U SolarinI; F O NjokanmaII
IFWAC (Paed), Cert Nephrology (SA) (Paed); Renal (ISN/IPNA) Fellow, Department of Paediatric Nephrology, Red Cross War Memorial Children's Hospital, Cape Town, South Africa; Department of Paediatrics, Babcock University Teaching Hospital, Ilishan-Remo, Ogun State, Nigeria
IIFWAC (Paed), FMC (Paed); Department of Paediatrics, Lagos State University Teaching Hospital, Lagos, Nigeria
ABSTRACT
OBJECTIVE: To determine the sensitivity, specificity, and positive and negative predictive values of the Micral-Test in determination of microalbuminuria (MA).
METHODS: Eighty children aged 5 -15 years with sickle cell anaemia (SCA) (HbSS) in a steady state attending the Lagos State University Teaching Hospital were recruited. The subjects were age-, sex- and social-class-matched with controls of genotype AA (HbAA). This prospective, descriptive and cross-sectional study lasted for 3 months, between October and December 2009. Morning random spot urine was used to assess the Micral-Test and the albumin/creatinine ratio (ACR). The performance of the Micral-Test was determined using the ACR as the gold standard.
RESULT: The sensitivity and specificity of the Micral-Test were 84.6% and 81.0%, respectively. The sensitivity and specificity were 100% and 86.6%, respectively, in children <10 years of age compared with 80% and 73.8%, respectively, in those >10 years old. The positive predictive value was 28.2% and the negative predictive value was 98% among the study subjects.
CONCLUSION: The sensitivity and specificity of the Micral-Test make it a good screening tool to detect MA in children with SCA. The Micral-Test is cheaper than quantitative measurement of ACR. Patients with a single positive Micral-Test should be followed up with two more Micral-Tests over a 3-month period to confirm persistent MA.
Sickle cell anaemia (SCA) accounts for more than three-quarters of the >300 000 children born worldwide with a serious Hb disorder.[1] SCA is inherited as an autosomal recessive disorder and is the most common inherited condition affecting red blood cells among blacks. There is a high burden of the disease in Africa, particularly in Nigeria, where the heterozygous (AS) carrier rate is ~25% and homozygous rate (SS) is ~3%.[2,3] SCA accounts for 5% of <5-year-old deaths on the African continent, more than 9% of such deaths in West Africa and up to 16% in individual West African countries.[4] The disease contributes to up to 26.5% of deaths in children aged 5 - 15 years in Nigeria; this high disease burden makes SCA a major cause of mortality in Nigeria.[5,6]
SCA is associated with alterations in the functions of many organ systems, including the kidney.[7] Renal abnormalities associated with SCA include concentration defects, impaired urinary acidification, disruption of medullary vasculature, cortical scarring, proteinuria and uraemia.[7] Renal failure, a major complication, has been found to affect 5 - 18% of all patients with SCA, and leads to early death.[8] Derangement of renal function observed in SCA is partly explained by hyperfiltration and hyperperfusion. Essentially, these two processes are consequences of significant insult to the glomerular basement membrane, resulting in excessive loss of albumin in the urine. This glomerulopathy is responsible for proteinuria and progressive renal insufficiency.[9,10] An albumin excretion rate of <20 μg/min (i.e. 30 mg/24 hours) is considered normal in healthy individuals.[11] However, in SCA, early in the process of altered renal function, albumin is lost in the urine in minute quantities above the acceptable range but below the level (30 mg/dL) detectable by routine dipstick analysis. The excretion of proteins by the kidneys above the 'normal' range but below the level of standard dipstick detection is termed microalbuminuria (MA).[12] MA is a prelude to the development of overt proteinuria.
The use of MA in the prediction of future development of overt renal disease in diabetes and hypertension has been established in diseases such as diabetes and hypertension. Methods employed include the 24-hour urine albumin test, the overnight urine albumin test (these are timed collections) and the spot urine test for albumin and/or albumin/creatinine ratio (ACR) (these are early-morning or random urine tests). The most accurate microalbumin measurement is the 24-hour urine test, but this is cumbersome in children and relies on patient compliance, which may be limited. When urinary creatinine measurement is performed along with a spot microalbumin test, the resulting ACR approaches the accuracy of the 24-hour microalbumin test without the extended collection difficulties.[10] An alternative to the 24-hour microalbumin test is the spot microalbumin test (spot urine). The ease of specimen collection, performance of the test and universal acceptance of results have made this alternative attractive.[10]
The objective of the current study was to determine the usefulness (accuracy) of a spot test (Micral-Test) compared with ACR as the gold standard.
Methods
This study was conducted between October and December 2009 at the Sickle Cell Clinic of the Lagos State University Teaching Hospital, Lagos, Nigeria. The subjects included 80 children aged 5 - 15 years with Hb genotype SS (HbSS), matched for age and sex with 80 children with Hb genotype AA (HbAA). Equal numbers of boys and girls were recruited. The number of subjects recruited from each age group depended on the proportions of the different age groups of patients on the clinic register.
Hb genotype was determined by electrophoresis using cellulose acetate paper. Both subjects and controls tested negative for MA by dipstick urinalysis. Subjects were in a steady state at the time of investigation, defined as the absence of fever, acute illness or crisis in the previous 4 weeks or more, and taking no other medication than routine folic acid and prophylactic antimalarial medication.[13]
Written informed parental consent and assent of the subject, where applicable, and Institutional Ethics Review Board approval were obtained before commencement of the study.
Sample size was calculated using the formula for determining single proportions.[14] The assumed prevalence of MA from a previous study was 19.2%.[15] An effort was made to ensure an even age and gender representation in the sample.
The following subjects were excluded from the study: children with symptoms and signs suggestive of urinary tract infection or pre-existing renal disease, children with ongoing menstruation or vaginal/penile discharge, and children with a history of exposure to drugs such as oxytetracycline within 72 hours of the study.
For each child, a detailed history was obtained, including age, sex and relevant medical history including recurrent admissions, blood transfusions, cigarette smoking, family history of hypertension, and drugs taken within the preceding 72 hours. Each child was awarded a socioeconomic index based on the occupations and educational attainment of parents or their caregivers, using the method described by Oyedeji.[16] Those in social classes I and II were regarded as in the upper socioeconomic stratum, those in social class 3 were regarded as in the middle socioeconomic stratum, while those in social classes IV and V were in the lower socioeconomic stratum.
A thorough physical examination was carried out on each child. Axillary temperature was recorded in degrees Celsius (°C). A child was regarded as febrile if the temperature was >37.5°C.[17] Weight and height were recorded, and body surface area (BSA) and body mass index (BMI) were derived using standard formulae.[18] The blood pressure (BP) reading was taken on arrival at the clinic and only repeated on departure if the initial reading was very high. This was to minimise white-coat hypertension. The BP was recorded using an Accoson (UK) sphygmomanometer using standard methods.[19] Hypertension was defined as an average systolic BP that was >95th percentile for sex, age and height.[19]
Specimen collection
All subjects were provided with three universal bottles for the collection of morning random urine samples: one bottle for macroalbuminuria using Combi-screen strips (combi-10 multistrips) and the other two bottles for the Micral-Test and ACR, respectively. Only subjects whose Combi-screen results were negative were evaluated for MA by Micral-Test and microalbumin/creatinine quantification. Caregivers and the subjects were instructed on how to collect a midstream urine sample. The morning urine samples for ACR were sent to the diagnostic laboratory within 2 hours of collection.
About 2 mL of venous blood were collected from each patient by venepuncture, using aseptic procedure. The blood sample was for confirmation of Hb genotype, which was determined by paper electrophoresis using tris buffer at pH 8.6.[20]
Urine specimens were screened for MA using Micral-Test strips (Roche Diagnostics, USA). The urine samples were tested according to manufacturer's instructions. Screening was considered positive when values of >20 mg/L were obtained.
Urine microalbumin was measured using the MA reagent in conjunction with Synchron CX System and Synchron CX MA Calibrator (Beckman Coulter, USA) in our diagnostic laboratory. The MA reagent was used to measure the albumin concentration by a turbidimetric method. Urine creatinine was measured using the alkaline picrate method.[21] Urine ACR was mathematically derived.
Ratios of 30 - 300 mg/g were classified as MA. Based on the results of the urine screening, children were classified as follows: MA absent, i.e. urine microalbumin <30 mg/g, or MA present, i.e. urine microalbumin 30 - 300 mg/g.
Statistical analysis
Data for 160 subjects (80 sickle cell patients and 80 controls) were analysed using SPSS version 16.0 (IBM, USA). Descriptive statistics (means and standard deviations (SDs)) were calculated for continuous variables. Differences between mean values were evaluated using Student's i-test, while discrete variables were compared using χ2 tests or odds ratios at 95% confidence limits.
Results
A total of 160 children comprising 80 subjects with HbSS and 80 HbAA controls were recruited into the study. Table 1 shows the distribution of subjects and controls according to age, gender and socioeconomic class. By design, equal numbers of males and females were recruited. The mean (SD) age of HbSS subjects was 9.58 (3.26) years, and of the controls was 9.62 (3.40) years (p=0.988). The majority of families were of the upper and middle social strata, both accounting for >80% of either HbSS subjects or HbAA controls. Overall, HbSS subjects weighed significantly less than controls (p=0.001). Similarly, the mean height (131.20 (16.12) cm), mean body surface area (BSA) (0.9 (0.26) m2) and mean BMI (14.80 (2.04) kg/m2) of HbSS subjects were significantly lower compared with HbAA controls (136.30 (16.01) cm, 1.1 (0.28) m2 and 17.40 (5.23) kg/m2 for height, BSA and BMI, respectively) (p=0.045, p<0.001 and p<0.001, respectively). The mean systolic BP was comparable in the two groups (99.4 (12.7) mmHg for HbSS and 98.8 (12.3) mmHg for HbAA, p=0.763). The diastolic BP was lower in the SCA subjects (98.80 (12.28) mmHg) compared with that of the controls (60.74 (10.66) mmHg, p=0.001).
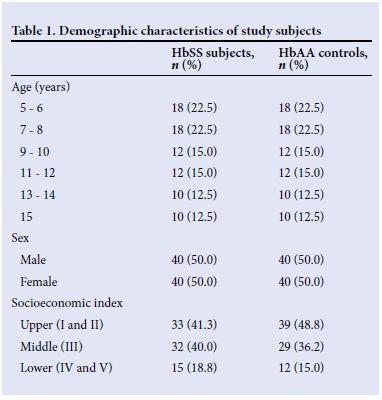
Percentages are of total number of HbSS subjects (n=80) or HbAA controls (n=80), as applicable.
Concordance of Micral-Test and ACR
ACR was used as the gold standard and the Micral-Test as the screening test at this phase. All subjects, HbSS and HbAA, were pooled and analysed as a group. The sensitivity, specificity, positive predictive value and negative predictive value of the Micral-Test were 84.6%, 81.0%, 28.2% and 98.3%, respectively.
Next, the concordance analyses were repeated according to age groups (<10 years and >10 years) (Tables 2 and 3, respectively). Sensitivity, specificity and negative predictive value of the Micral-Test were higher in younger subjects, while positive predictive value was higher in older subjects. In spite of observed differences, sensitivity of the Micral-Test remained >80%, specificity >70% and negative predictive value >95%, irrespective of age group. Also, positive predictive value of the Micral-Test was <40% for both age groups.


Discussion
The current study demonstrated high performance of the Micral-Test as a screening test, using ACR as the reference standard. The high sensitivity and specificity rates of the Micral-Test for MA (84.6% and 81.0%, respectively) fall within the limits claimed by the manufacturers and corroborate the findings of earlier clinical studies.[22] Mogensen et al.,[23] in a large multicentre study, observed a sensitivity of 96.7% and a specificity of 71% for the diagnosis of MA with the Micral-Test II. The reference standard for MA diagnosis was the measurement of albumin concentration in a spot urine sample (20 mg/L) rather than ACR determination. Incerti et al.[24] demonstrated the sensitivity and specificity of the Micral-Test as 90% and 46%, respectively, using urine albumin excretion rate in 24-hour urine as the reference standard on the receiver operating characteristic curve. A study in the USA found a sensitivity of 88% and specificity of 80%, which are similar to the current study;[12] however, our study observed a lower positive predictive value of 28.2% than the 69% reported in their study.
Other authors have also described a higher sensitivity (95.2%) and specificity (84.7%) for MA diagnosis than those observed in the current study.[25] These differences could be due to the performance of strip readings in first morning urine specimens, in which the known diurnal variation in albumin excretion is not present, compared with a random urine specimen. Albumin measurements in a first morning urine specimen correlate more with 24-hour protein excretion than measurements in a random spot urine sample.[26,27]
A good screening test is expected to have a very high sensitivity so that very few true positives are missed. This requirement is demonstrated by the Micral-Test, with 84.6% sensitivity. In addition, the high specificity of 81% and high negative predictive value of 98.3% show the ability of the Micral-Test to identify true negatives and to exclude false negatives. Perhaps the strongest finding with the use of the Micral-Test in this study is the very high negative predictive value of 98.3%. The implication is that in negative cases, the test result is almost uniformly correct.
The main limitation of the Micral-Test is its low positive predictive value of 28.2%. This implies that if a test is positive, the patient would need further evaluation to be certain that it is not a case of false positivity. However, the impressive diagnostic indices of the Micral-Test for MA remained high irrespective of age of subjects. Thus, overall, the value of the Micral-Test in the investigation of MA is very good.
The average cost of performing the Micral-Test was USD5(NGN750) per subject, while ACR cost about USD30 (NGN4 200) per test. It is important to note that quantitative ACR can only be done where there is suitable laboratory structure, whereas the Micral-Test does not depend on any prerequisite. In this regard, immediate urine results using the Micral-Test may represent an advantage, especially when a standard quantitative technique is not readily available. According to National Kidney Foundation/Kidney Disease Outcome Quality Initiative (NKF/KDOQI) guidelines,[27] it is usually not necessary to obtain a timed urine collection for MA evaluation, and albumin should be measured in a spot urine sample using either an albumin-specific dipstick or ACR. However, it is important to note that patients with a positive dipstick test should undergo confirmation of MA by a quantitative measurement.[27]
Renal failure, a major complication of sickle cell disease, leads to early death.[5] Scheven et al.,[28] in their recent study, identified isolated MA as indicative of a poor medical prognosis. Early detection of the at-risk population of SCA and forestalling progression of disease are important. With the high burden of SCA and the seemingly bleak future for curative nephrology in the resource-poor Nigerian environment, prevention is definitely key.
Conclusion
The sensitivity and specificity of the Micral-Test makes it a good screening tool to detect MA in children with SCA. The Micral-Test is cheaper than the quantitative measurement of ACR. Patients with a single positive Micral-Test should be followed up with two more Micral-Tests over a 3-month period to confirm persistent MA. Our study was a cross-sectional study, therefore we cannot say how many sickle cell subjects have ended up in renal failure and how long after the identification of MA. A longitudinal study would be required to answer these questions.
References
1. Akinyanju O, Ohujohungbe A. How to Live with Sickle Cell Disorder. Ibadan, Book Builders Edition Africa, 2006. [ Links ]
2. Akinyanju, OO. A profile of sickle cell disease in Nigeria. Ann N Y Acad Sci 1998;565:126-134. [ Links ]
3. Adekile AD. Haemoglobinopathies. In: Azubuike JC, Nkanginieme KEO, eds. Paediatric and Child Health in a Tropical Region. 2nd ed. Owerri: African Education Services, 1999;194-312. [ Links ]
4. World Health Organization. Sickle cell anaemia. www.who.int/gb/ebwha/pdf_files/wHA (accessed 12 October 2010). [ Links ]
5. Adeyokunnu AA, Taiwo A, Antia AU. Childhood mortality among 22 225 consecutive admissions in the University College Hospital Ibadan. Niger J Paediatr 1980;7(1):7-15. [ Links ]
6. Fagbule D, Joiner KT. Pattern of childhood mortality at the University of Ilorin Teaching Hospital. Niger J Paediatr 1987;14(1):1-5. [ Links ]
7. Searjeant GR. Sickle cell disease. 2nd ed. Oxford: Oxford Medical Publications, 1992:261-281. [ Links ]
8. McBurney PG, Hancvold CD, Hernandez CM, Waller JL, Mckie KM. Risk factors for microalbuminuria in children with sickle cell anaemia. J Pediatr Hematol Oncol 2002;24(6):473-477. [ Links ]
9. Alvarez O, Montane B, Lopez G, Wilkinson J, Miller T. Early blood transfusion protects against microalbuminuria in children with sickle cell disease. Pediatr Blood Cancer 2006;47(1):71-6. [http://dx.doi.org/10.1002/pbc.20645] [ Links ]
10. De Jong PE, Curhan GC. Screening, monitoring and treatment of albuminuria: Public health perspectives. J Am Soc Nephrol 2006;17(8):2120-2126. [http://dx.doi.org/10.1681/ASN.2006010097] [ Links ]
11. Rowe DJ, Bagga H, Betts PB. Normal variation in rate of albumin excretion and albumin to creatinine ratios in overnight and daytime urine collection in non-diabetic children. Br Med J (Clin Res Ed) 1985;291(6497):693-694. [ Links ]
12. Mogensen CE, Friedman C. Clinician's Manual on Microalbuminuria. London: Current Medical Group, 2006. [ Links ]
13. Ojuawo A, Adedoyin MA, Fagbule D. Hepatic function test in children with sickle cell anaemia during vaso-occlusive crisis. Central Afr J Med 1994;40(12):342-345. [ Links ]
14. Bland JM, Butland BK, Peacock JL, Poloniecki J, Reid F, Sedgwick P. Statistics guide for research grant applicants. http://www.sgul.ac.uk/depts/chs/discipline-groups/statguide/size.cfm (accessed 2 December 2008). [ Links ]
15. Datta V, Ayengar JR, Karpate S, Chaturredi P. Microalbuminuria as a predictor of early glomerular injury in children with sickle cell disease. Indian J Paediatr 2003;70(4):307-309. [http://dx.doi.org/10.1007/BF02723586] [ Links ]
16. Oyedeji GA. Socio-economic and cultural background of hospitalized children in Ilesha. Niger J Paediatr 1985;12(4):111-117. [ Links ]
17. Mato CN, Anochie IC, Uchenna DI, Ikimalo JI. Introduction to clinical medicine: An objective-based learning manual. Port Harcourt: University of Port Harcourt Press, 2007:74. [ Links ]
18. Mosteller RD. Simplified calculation of body surface area. N Engl J Med 1987;317(17):1098. [ Links ]
19. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics 2004;114(2 Suppl 4th Report):S555-S556. [ Links ]
20. Nicholson JF, Pesce MA. Reference ranges for laboratory tests and procedures. In: Behrman RE, ed. Nelson Textbook of Paediatrics. 16th ed. Philadelphia: WB Saunders Co, 2000:2182. [ Links ]
21. Knox-Macaulay HM. Molecular biology and inheritance. In: Fleming AF, ed. Sickle Cell Disease: A Handbook for the General Clinician. Edinburgh: Churchill Livingstone Inc, 1982:20. [ Links ]
22. Marouf R, Mojiminiyi O, Abdella N, Kortom M, Al Wazzan H. Comparison of renal function markers in Kuwaiti patients with sickle cell disease. J Clin Pathol 2006;59(4):345-351. [http://dx.doi.org/10.1136/jcp.2005.026799] [ Links ]
23. Mogensen CE, Viberti GC, Peheim E, et al. Multicenter evaluation of the Micral-Test II test strip, an immunologic rapid test for the detection of microalbuminuria. Diabetes Care 1997;20(11):1642-1646. [ Links ]
24. Incerti J, Zelmanovitz T, Camargo JL, Gross JL, de Azevedo MJ. Evaluation of tests for microalbuminuria screening in patients with diabetes. Nephrol Dial Transplant 2005;20(11):2402-2407. [http://dx.doi.org/10.1093/ndt/gfi074] [ Links ]
25. Lepore G, Maglio ML, Nosari I, Dodesini AR, Trevisan R. Cost-effectiveness of two screening programs for microalbuminuria in type 2 diabetes. Diabetes Care 2002;25(11):2103-2104. [ Links ]
26. Molitch ME, DeFronzo RA, Franz MJ, et al., American Diabetes Association. Nephropathy in diabetes. Diabetes Care 2004;27(Suppl 1):S79-S83. [ Links ]
27. National Kidney Foundation/Kidney Disease Outcome Quality Initiative. NKF/KDOQI Guidelines. http://www2.kidney.org/professionals/KDOQI/guidelines_ckd/p5_lab_g5.htm (accessed 19 September 2013). [ Links ]
28. Scheven L, Van der Velde M, Lambers Heersprink HJ, De Jong PE, Ganservoort RT. Isolated microalbuminuria indicates a poor medical prognosis. Nephrol Dial Transplant 2013;28(7):1794-1801. [http://dx.doi.org/10.1093/ndt/gft031] [ Links ]
 Correspondence:
Correspondence:
A U Solarin
asolar234@gmail.com














