Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.9 no.1 Pretoria Jan. 2015
RESEARCH
Ventriculostomy infections at the paediatric neurosurgical unit at Dr George Mukhari Academic Hospital
D P MotlobaI; M D NgqanduII
IBDS, MDent (Comm Dent), MPH (Epidemiology), MBL; Department of Community Dentistry, University of Limpopo, Medunsa Campus, Pretoria, South Africa
IIMB ChB, MMed (Neurosurgery); Department of Neurosurgery, University of Limpopo, Medunsa Campus, Pretoria, South Africa
ABSTRACT
BACKGROUND: External ventricular drains (EVDs) are essential to the clinical management and care ofpatients with neurosurgical complications, but EVD use is routinely associated with concomitant infection, sometimes resulting in mortality
OBJECTIVE: To undertake an epidemiological study of ventriculostomy-related infections among paediatric neurosurgical patients at the Dr George Mukhari Academic Hospital, Pretoria, South Africa
METHODS: Retrospective analysis was conducted on the clinical records of 92 children admitted to the neurosurgical unit at the hospital between 2010 and 2013. Records were included in the study only if they were complete, legible and accurate. Data were collected on the following variables: age, gender, frequency of catheter change, cerebrospinal fluid (CSF) sampling, use of prophylaxis, microbiology, Glasgow Coma Scale, glucose, chlorine, and other clinical, chemical and laboratory parameters routinely observed as part of patients' work-ups. Results. Two or more EVDs were placed on 45.7% (40) children, with a maximum of seven EVDs per child. Ventriculitis incidence was 28.3% (26 of 92). There was a significant association between the number of EVDs inserted and the incidence of ventriculitis (p=0.010). More frequent CSF sampling also increased ventricular-related infections (p=0.000), as did prolonged EVD retention (p=0.001). Using prophylactic antibiotics or impregnated catheters did not reduce ventriculitis incidence significantly
CONCLUSION: Evidence supports adherence to strict sterilisation protocols and techniques when inserting EVDs. Catheters should not be retained for extended periods, and CSF sampling can be limited to once in 3 days. Routine use of antibiotic-impregnated EVDs and antistaphylococcal prophylaxis is still recommended
External ventricular drains (EVDs) are regarded as an essential part of the armamentarium available to divert pathological and excess cerebrospinal fluid (CSF) from the ventricles to the exterior in a variety of neurosurgical conditions. Despite their proven success and efficiency, EVDs have been associated with increased incidence of ventriculitis.
Ventriculitis forms part of a wide clinical spectrum that includes ventricle-associated infection and ventriculostomy colonisation as part of the same disease phenomenon. A lack of consensus about the exact definition of ventricular infections accounts for a wide range in the incidence of ventriculitis reported (0 - 45%), possibly due to misclassification bias.[1,2]
The literature is inconsistent about the determinants of ventriculitis, especially in paediatric patients, on whom data are relatively scarce. Most studies associate increased incidence of EVD-related infections with lack of sterility, catheter duration, number of EVDs used, catheter-type EVDs, lack of prophylactic antibiotic use, colonisation and systemic infection.[3] Gram-negative pathogens are largely implicated, more so than Gram-positive pathogens, and coagulase-negative Staphylococcus spp. are the most frequently reported colonising bacteria. Thus far, there is no consensus on the effectiveness of using antibiotic-impregnated catheters and prophylaxis to reduce infections.
Against this background, we conducted this research at the paediatric neurosurgery unit of the Dr George Mukhari Academic Hospital at the Medunsa Campus of the University of Limpopo, Pretoria, South Africa (SA), to estimate the incidence of ventriculitis in this unit; no prior study of this nature had been done (in general, similar studies in paediatric populations are limited, particularly in SA). The specific research objectives of the study included exploring the distribution and determinants of ventriculitis, and estimating associations with some clinical and demographic indicators.
Methods
A descriptive survey of clinical records was selected as the study design, because it is appropriate and convenient for this setting and purpose. Data were collected retrospectively from the records of patients admitted to the paediatric neurosurgical unit at the hospital between January 2010 and December 2013, who had had an EVD inserted as part of their treatment, and if all records were accurate, legible and complete. Patients were excluded from the study if they were older than 12 years. Information was sourced from the hospital's clinical records, specifically from theatre records and the neurosurgical unit, and the National Health Laboratory Service.
Data were collected on each patient's age, gender, type of EVD, number of EVD changes, duration for which the EVD was in situ, the frequency of CSF sampling, prophylactic antibiotic use and outcome. For the purposes of our study, ventriculitis was defined as a positive culture (based on a CSF sample or a sample from the tip of the catheter), together with a systemic manifestation of infection, such as fever and/or high C-reactive protein.
Statistical analysis
Biostatistical analysis was conducted using SPSS version 22 (IBM, USA). Hypothesis testing was done to establish any association between the incidence of ventriculitis and the descriptive variables, by using Pearson's χ2 test for categorical variables and Student's t-test for continuous variables. The p-values associated with the tests were considered significant at p<0.05.
Ethical approval
This study was reviewed and approved by the Medunsa Research Ethics Committee (MREC/M/291/2012: PG).
Results
Over the review period of 3 years, 92 patients had EVDs inserted during hospitalisation. Of the 92 patients, 26 (28.3%) developed ventriculitis. A further seven cases showed bacteriological growth on CSF culture, but were considered to be colonised or contaminated on additional clinical grounds.
Patient demographics
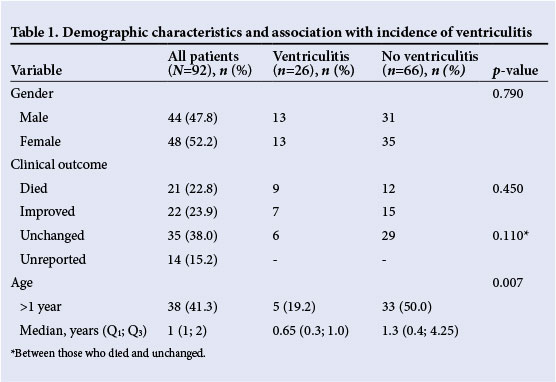
The results reflected no gender predisposition to develop ventriculitis in this population (p=0.79). However, we found a significant negative association between age and ventriculitis (p=0.007): as age increased, the incidence of ventriculitis decreased. Clinical outcomes were reported on 78 children, of whom 21 (22.8%) died, and 22 (23.9%) recovered fully. Notably, children who developed ventriculitis were 1.6 times more likely to die than to recover, but this finding was not statistically significant (p=0.45) (Table 1).
Attributes of EVDs and incidence of ventriculitis
Children who developed ventriculitis had two times more EVDs inserted than non-cases; the difference was significant (p=0.01). Increased frequency of CSF collection significantly increased the incidence of ventriculitis (p=0.00). Increased length of time for which EVDs were kept in situ also significantly increased the incidence of ventriculitis (p=0.001). The length of time for which EVDs were in situ was five times as long in those who developed ventriculitis as in those who did not (Table 2).

Antibiotic use and ventriculostomy infection
Prophylactic antibiotic cover and catheter types were used based on availability. With this pattern of use, it was found that prophylactic antibiotic cover did not reduce the risk of infection. Similarly, the incidence of ventriculitis remained similar between groups of children for whom antibiotic-impregnated catheters were used (42%) v. groups of children for whom standard, plain EVDs were used (37.5%). However, the results were not statistically significant (Table 3).

Of the samples processed, 26.1% (24 of 92) revealed bacterial growth, characterised by slightly more Gram-negative microorganisms. Coagulase-negative Staphylococcus, which is commonly regarded as a contaminant, was isolated in 43.5% (10 of 24) of the samples, Pseudomonas aeruginosa was found in 21.7%, and Klebsiella pneumoniae in 17.4% (Table 4). The risk of developing ventriculitis was three times higher from Gram-negative organisms than from Gram-positives, which supports the assertion that Gram-negative microorganisms are primary causative agents in ventriculitis (p=0.008) (Table 5).


Discussion
The incidence of ventriculitis in our study was 28.3%, higher than the results reported in prior studies, namely 6.65%,[3] 0 - 22%,[4] and 23.3%.[5] This variance in infection rates may be attributed to the lack of a uniform definition of ventriculitis, which renders comparison of findings difficult.[1] In our study, a stringent definition of ventriculitis (incorporating positive cultures, CSF pleocytosis and clinical symptoms) was applied in order to reduce the possibility of misclassification.[6]
Our finding that gender was not correlated with ventriculitis supports the results reported by Kim et al.[6] and Lyke et al.,[7] but is contrary to the findings of Lo et al.[8] and Arabi et al.,[9] who found that more female than male patients developed ventriculitis. While gender as a risk factor for disease is well-established in older cohorts, we did not find that gender-related variations mediated or played a role in infection in children.
Because most studies focus on adults, it is difficult to show any relationship between ventriculitis and age. However, our results were based on a paediatric population, and they revealed a significant negative association between age and ventriculitis. So far, very few studies support our findings that there is a significant age-related risk and susceptibility to the development of ventriculostomy-related infections.[10,11] This strong association emphasises the need to institute stringent clinical and preventative measures among the youngest children, who face an increased risk of infection.
The statistically significant association between how long EVDs are kept in situ and the onset of ventriculitis is similar to the findings of other studies.[9,11,12] Specific evidence reported in the literature suggests that the risk of infection increases when EVDs are retained for more than 5 days,[11] 7 days[9] and 10 days.[13] Our results indicated that multiple catheterisation or frequent EVD changes are associated with high infection rates; similar outcomes are reported in several other studies.[14,15] However, in contrast, some authors assert that multiple EVD replacements are not a significant independent risk factor for ventriculitis.[13,15]
CSF sampling poses a risk of infection: Korinek et al.[16] and Hoefnagel et al.[10] have demonstrated that serial collection of CSF tended to predispose patients to ventriculitis. Our findings support such an association, because more than twice the number of CSF samples were collected from those who developed ventriculitis than from those who did not, and the difference was statistically significant. Collectively and individually, multiple CSF sampling and catheterisation contributed to an increased risk of bacterial contamination and colonisation. Concomitantly, prolonged retention of EVDs promotes microbiological shift, bacterial succession and increased virulence the longer the EVD remains in place. This may explain the increased incidence of ventriculitis associated with these factors.
Debate continues about the efficacy of antibiotic-impregnated catheters in reducing EVD-related infection.[16] Pooled metaanalyses confirm the positive affect of antibiotics in reducing ventriculitis.[16,17] Our findings (p=0.797) in this regard showed insignificant differences and therefore support similar reports of no association.[18] However, failure to demonstrate this relationship may be attributed to the small sample size, and the less robust, retrospective, cross-sectional study design.
In line with our findings, microorganisms derived from the CSF or catheters were predominately Gram-negative. Coagulase-negative Staphylococcus is commonly introduced by EVD insertion, because it is predominantly skin commensal.[16]
Study limitations
A retrospective study design and the small sample size could have a negative effect on the validity and generalisation of the results. Other limitations are the lack of data on the patients' HIV status and indications for antibiotic cover. Despite these limitations, this study provides credible contribution to the existing body of knowledge.
Conclusion
We conclude that prolonged retention of EVDs, increased frequency of EVD changes and increased CSF sampling increase the incidence of infection. The use of prophylactic antibiotics and antibiotic-impregnated catheters may reduce the occurrence of ventriculitis, although the evidence is not significant.
We recommend that catheters be retained for maximum 5 days, that CSF sampling be limited to once every 3 days unless dictated by clinical circumstances, and that strict sterilisation protocols and thorough aseptic techniques be adhered to during insertion EVD insertion. In addition, antibiotic-impregnated EVDs should be used and antistaphylococcal prophylaxis should be administered with EVD insertion.
References
1. Kim DK, Uttley D, Bell BA, Marsh HT, Moore AJ. Comparison of rates of infection of two methods of emergency ventricular drainage. J Neurol Neurosurg Psychiatry 1995;58(4):444-446. [ Links ]
2. Dasic D, Hanna SJ, Bojanic S, Kerr RS. External ventricular drain infection: The effect of a strict protocol on infection rates and a review of the literature. Br J Neurosurg 2006;20(5):296-300. [http://dx.doi.org/10.1080/02688690600999901] [ Links ]
3. Lozier AP, Sciacca RR, Romagnoli MF, Connolly, ES Jr. Ventriculostomy-related infections: A critical review of the literature. Neurosurgery 2002;51(1):170-181. [http://dx.doi.org/10.1097/00006123-200207000-00024] [ Links ]
4. Lee JH, Cha SH, Lee J, et al. Ventriculostomy-related infections in the neurosurgical intensive care unit: The risk factors and the outcomes. Korean J Crit Care Med 2011;26(4):208-211. [http://dx.doi.org/10.4266/kjccm.2011.26.4.208] [ Links ]
5. Strojnik T, Golc J, Zakelsek J. Infections of external ventricular drainages. Cent Eur J Med 2013;8(2):250-256. [http://dx.doi.org/10.2478/s11536-012-0115-8] [ Links ]
6. Kim JH, Desai NS, Ricci J, et al. Factors contributing to ventriculostomy infection. World Neurosurg 2012;77(1):135-140. [http://dx.doi.org/10.1016/j.wneu.2011.04.017] [ Links ]
7. Lyke, KE, Obasanjo OO, Williams MA, O'Brien M, Chotani R, Perl TM. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin Infect Dis 2001;33(12):2028-2033. [http://dx.doi.org/10.1086/324492] [ Links ]
8. Lo CH, Spelman D, Bailey M, Cooper DJ, Rosenfeld JV, Brecknell JE. External ventricular drain infections are independent of drain duration: An argument against elective revision. J Neurosurg 2007;106(3):378-383. [http://dx.doi.org/10.3171/jns.2007.106.3.378] [ Links ]
9. Arabi Y, Memish ZA, Balkhy HH, et al. Ventriculostomy-associated infections: Incidence and risk factors. Am J Infect Control 2005;33(3):137-143. [http://dx.doi.org/10.1016/j.ajic.2004.11.008] [ Links ]
10. Hoefnagel D, Dammers R, Ter Laak-Poort MP, Avezaat, CJ. Risk factors for infections related to external ventricular drainage. Acta Neurochir (Wien) 2008;150(3):209-214. [http://dx.doi.org/10.1007/s00701-007-1458-9] [ Links ]
11. Park P, Garton, HJL, Kocan, MJ, Thompson, BG. Risk of infection with prolonged ventricular catheterization. Neurosurgery 2004;55(3):594-601. [ Links ]
12. Kitchen WJ, Singh N, Hulme S, Galea J, Patel HC, King AT. External ventricular drain infection: Improved technique can reduce infection rates. Br J Neurosurg 2011;5(5):632-635. [http://dx.doi.org/10.3109/02688697.2011.578770] [ Links ]
13. Holloway, KL, Barnes T, Choi S, et al. Ventriculostomy infections: The effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg 1996;85(3):419-424. [ Links ]
14. Pople I, Poon W, Assaker R, et al. Comparison of infection rate with the use of antibiotic-impregnated v. standard extraventricular drainage devices: A prospective, randomized controlled trial. Neurosurgery 2012;71(1):6-13 [http://dx.doi.org/10.1227/NEU.0b013e3182544e31] [ Links ]
15. Zabramski, JM, Whiting, D, Darouiche, RO, Horner, TG, Olson, J, Robertson C. Efficacy of antimicrobial-impregnated external ventricular drain catheters: A prospective, randomized, controlled trial. J Neurosurg 2003;98(4):725-730. [http://dx.doi.org/10.3171/jns.2003.98.4.0725] [ Links ]
16. Korinek AM, Reina M, Boch AL, Rivera AO, de Bels D, Puybasset L. Prevention of external ventricular drain-related ventriculitis. Acta Neurochir (Wien) 2005;147(1):39-45. [http://dx.doi.org/10.1007/s00701-004-0416-z] [ Links ]
17. Sonabend AM, Korenfeld Y, Crisman C, Badjatia N, Mayer SA, Connolly, ES Jr. Prevention of ventriculostomy-related infections with prophylactic antibiotics and antibiotic-coated external ventricular drains: A systematic review. Neurosurgery 2011;68(4):996-1005.[http://dx.doi.org/10.1227/NEU.0b013e3182096d84] [ Links ]
18. Gleicher D, Barad DH. Gender as a risk factor for autoimmune diseases. J Autoimmun 2007;28(1):1-6. [http://dx.doi.org/10.1016/j.jaut.2006.12.004] [ Links ]
 Correspondence:
Correspondence:
D P Motloba
pagollangmotloba@hotmail.com














