Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.8 no.4 Pretoria Nov. 2014
http://dx.doi.org/10.7196/SAJCH.752
RESEARCH
Characteristics and mortality rate of neonates with congenital cytomegalovirus infection
H A DiarI; S VelaphiII
IMB BCh, FC Paed, MMed; Division of Neonatology, Department of Paediatrics, Faculty of Health Sciences, University of the Witwatersrand, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
IIMB ChB, MMed; Division of Neonatology, Department of Paediatrics, Faculty of Health Sciences, University of the Witwatersrand, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Cytomegalovirus (CMV) infection is a common congenital infection in neonates. Clinical presentation and laboratory findings in CMV-infected infants in a setting where HIV is prevalent are not well characterised.
OBJECTIVE: To determine the characteristics and survival to hospital discharge of neonates with congenital CMV infection.
METHODS: In this retrospective, case-control study, hospital records of neonates, tested for CMV in the first 3 weeks of life from January 2004 to December 2008, were reviewed for maternal and neonatal characteristics, clinical presentation, laboratory findings and inpatient mortality. Comparisons were made between CMV-infected and CMV-uninfected neonates in those infants who were tested for CMV.
RESULTS: Among the CMV-infected, 91% were of low birth weight, 83% were preterm and 29% were small for gestational age. The CMV-infected neonates were more likely to present with hepato/splenomegaly compared with uninfected neonates (p=0.02). Thrombocytopenia was more severe in CMV-infected neonates (p=0.004). Congenital CMV-infected neonates were more likely to be HIV-exposed (p=0.003) and HIV-infected (p=0.02). Mortality before hospital discharge was significantly higher in congenital CMV-infected neonates (p=0.01) and in those with HIV co-infection (p=0.02). The male gender was a significant independent predictor of inpatient mortality (odds ratio: 23, 95% confidence interval 1.19 - 445.698; p=0.04).
CONCLUSION: Neonates presenting with hepato/splenomegaly and severe thrombocytopenia are most likely to be CMV-infected. Neonates with congenital CMV are more likely to be co-infected with HIV. The co-infection of CMV and HIV is associated with a high mortality rate, especially in male neonates.
Congenital cytomegalovirus (CMV) infection has been reported in as many as 23% of neonates born to mothers infected with HIV.[1] Congenital CMV infection has also been shown to be more common in HIV-infected infants than in HIV-uninfected infants, and to lead to a more rapid progression of HIV infection in infected infants.[1] Co-infection with CMV and HIV has been shown to occur in up to 50% of infants born to HIV-infected mothers.[2]
About 30% of mothers attending antenatal clinics in South Africa (SA) are HIV-positive.[2] A similar percentage has been noted among pregnant women attending antenatal care and/or delivering at Chris Hani Baragwanath Academic Hospital (CHBAH), a public government hospital. It is a common clinical practice in the neonatal unit of this hospital that neonates are investigated for CMV infection if born with thrombocytopenia, hepato/splenomegaly with or without thrombocytopenia and conjugated hyperbilirubinaemia. The number of patients presenting with the above clinical signs and laboratory findings have been noted to be increasing in parallel with the increase in HIV prevalence rate among pregnant mothers attending antenatal care in the hospital. Although all patients with hepato/splenomegaly and thrombocytopenia were investigated for CMV infection, not all of them had positive results. Therefore, in this study we compared CMV-infected and -uninfected neonates among those who were investigated and who had available laboratory results for CMV testing. Our hypothesis was that neonates with congenital CMV infection will have specific characteristics that identify them from those without congenital CMV infection. Congenital CMV infection was defined as a positive urine shell vial culture for CMV and/or a positive pp65 antigen in neonates tested before 21 days of life.
Methods
Study design
This was a descriptive study using a retrospective study design. Hospital clinical records of neonates who had specimens sent to the laboratory for urine shell vial culture and/or pp65 antigen test were reviewed.
Study population
The study sample included all patients who were admitted to the CHBAH neonatal unit and who had been investigated for suspected congenital CMV infection (i.e. who had urine shell vial culture or pp65 results) during the period from 1 January 2004 to 31 December 2008.
Setting
This study was conducted at CHBAH, a public government hospital in Johannesburg with 2 888 hospital beds, which serves the community of Soweto and is a referral centre for the southern part of Gauteng Province. Over the study period, the annual number of live births increased from 18 565 in 2004 to 22 849 in 2008. All sick neonates born in this hospital are admitted to the neonatal unit.
Study procedures
The names and hospital numbers of patients who had specimens processed and tested by the National Institute of Communicable Diseases (NICD) for pp65 antigen and/or shell vial culture were requested from NICD. Hospital records of these patients were retrieved and reviewed. Congenital CMV-infected neonates (pp65 and/or shell vial urine culture positive) were compared with congenital CMV-uninfected neonates among the tested neonates.
Data collection
The information collected included maternal and neonatal characteristics. The maternal characteristics included maternal age, parity, rapid plasma reagin (RPR) and HIV status. The neonatal characteristics included chronological age (days) at testing, gender, gestational age (weeks), weight (grams), length (centimetres) and head circumference (centimetres) at birth, neonatal clinical and laboratory data recorded in the hospital charts of the neonates at time of CMV testing and inpatient outcome.
Definitions
- Congenital CMV infection: positive urine shell vial culture and/or positive pp65 antigen test, within the first 3 weeks of life.
- Hepato/splenomegaly: the presence of hepatosplenomegaly, hepatomegaly or splenomegaly.
- Persistent thrombocytopenia: a platelet count <150 × 109/L reported on at least two consecutive blood tests.
- Leukopenia: a total white cell count of <5 × 109/L.
- Elevated alanine aminotransferase (ALT): ALT >25 U/L.
- Elevated aspartate aminotransferase (AST): AST >140 U/L.
- Elevated gamma-glutamyltransferase (γGT): γGT >132 U/L.3
Data capturing and analysis
Data was captured into a Microsoft Office Excel 2007 spreadsheet and statistical analysis was done using Statistica 9.1.210.0 and Stata/SE 12.0 for Windows (StataCorp LP, USA). In a normal and non-normal distribution, means (standard deviations (SDs)) and medians (interquartile ranges (IQRs)) were reported, respectively. The Student t-test and the Mann-Whitney U-test were used to compare continuous numerical variables. The χ2 test was used to compare two categorical variables. In all cases, p<0.05 represented statistically significant differences.
Ethical considerations
A coded number was allocated to each study patient to maintain confidentiality. The patients' identifiable factors were recorded and kept separately from the primary data for analysis.
Approval to perform this study was granted by the University of the Witwatersrand Human Research Ethics Committee (ethics clearance number: M080102) and the hospital protocol review committee.
Results
Descriptive statistics
During the 5-year study period from January 2004 to December 2008, a total of 177 neonates were tested for suspected CMV infection within the first 21 days of life; 28 were confirmed CMV cases (16%). Hospital records were retrieved for 86% (24/28) and 42% (62/149) of the patients who were confirmed to be congenital CMV-positive or CMV-negative, respectively (Fig. 1).
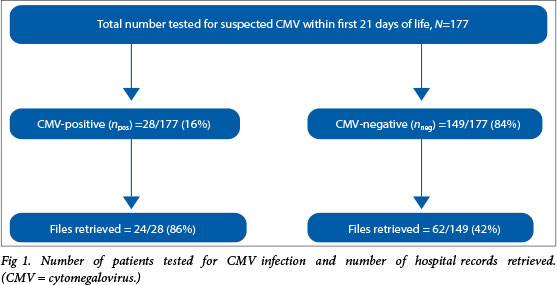
Congenital CMV infection among neonates
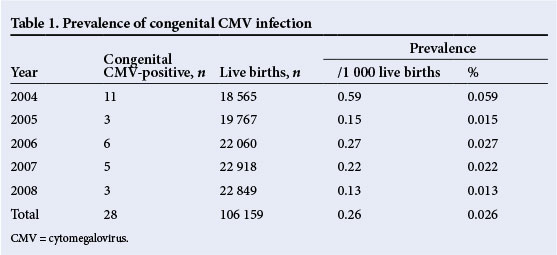
During the 5-year study period, the total number of live births was 106 159, with 28 neonates having confirmed congenital CMV infection, giving an incidence of 0.26/1 000 live births (range 0.13 - 0.59) or 0.026% (range 0.013 - 0.059%) (Table 1).
Maternal characteristics
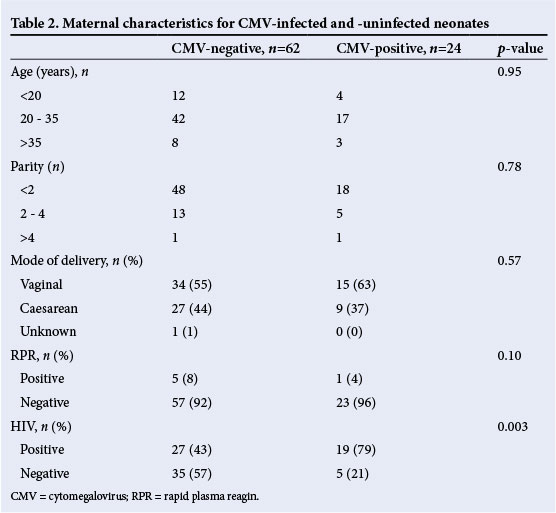
Table 2 shows the maternal characteristics for those neonates with congenital CMV infection. The categories are representative of teenage (13 - 19 years), child-bearing age (20 - 34 years) and advanced maternal age (≥35 years) pregnancies. The majority of pregnancies were in mothers of child-bearing age (71%) or mothers with less than three pregnancies (50%). Just over a third of patients were born by caesarian section. One patient was born to a mother who had a positive RPR. The majority of neonates with congenital CMV infection (19/24; 79%) were born to HIV-positive mothers. There were no statistical differences in maternal age, number of pregnancies, mode of delivery and maternal RPR results between the CMV-negative and CMV-positive neonates. The neonates who were CMV-positive were more likely to be born to mothers who were HIV-positive (HIV-exposed) (n=19/24 (79%) v. n=27/62 (44%); p =0.003).
CMV testing and demographic characteristics
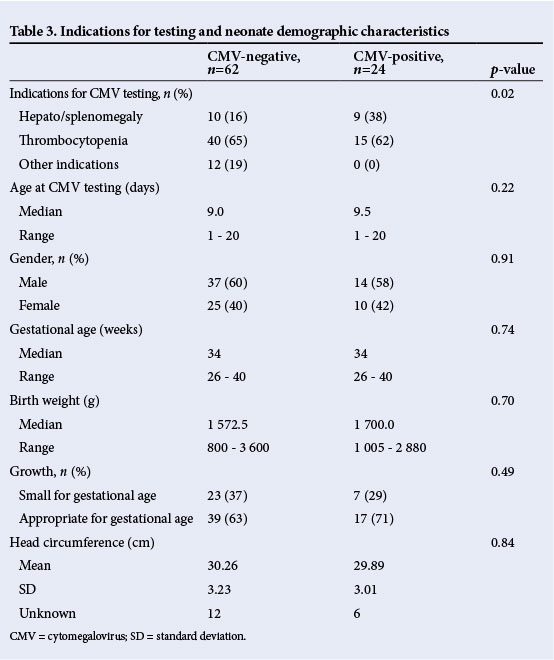
Among the neonates with congenital CMV infection, 92% had low birth weight, 83% were born preterm, 29% were small for gestational age (SGA) and 17% had microcephaly (Table 3). There were no significant differences between the incidence of SGA among the congenital CMV infected and uninfected (p=0.49). The common reasons for suspecting CMV were hepato/splenomegaly and thrombocytopenia. Other indications included jaundice, chronic lung disease, anaemia and leukopenia. The clinical presence of hepato/splenomegaly was more common in the CMV-infected infants than in the uninfected infants (p=0.02).
Complete blood counts and biochemical indices
Patients with CMV infection had thrombocytopenia (93%), and presented with direct hyperbilirubinaemia (42%), abnormal ALT concentration (42%) and/or abnormal γGT concentration (50%). Neonates in the congenital CMV-positive group were more likely to have lower platelet counts (p=0.004) compared with CMV-uninfected neonates. There were no significant differences for other laboratory parameters (Table 4).
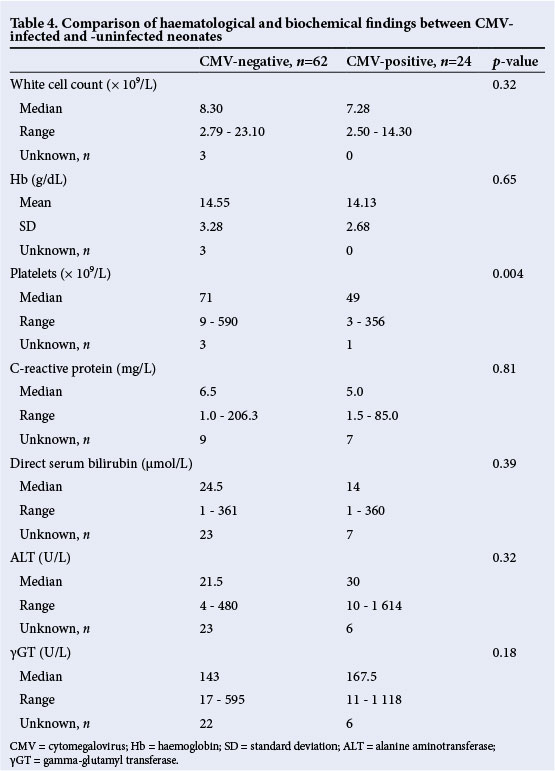
HIV exposure and infection
Table 5 reflects the neonatal HIV status based on HIV polymerase chain reaction (PCR) testing done at the chronological age of 6 weeks. The CMV-infected neonates had a high HIV transmission rate, with 13/19 who were HIV exposed being HIV-positive (HIV transmission rate of 68%), compared with 9/27 HIV exposed being HIV-positive (HIV transmission rate of 33%) in the CMV-uninfected group (p=0.02).
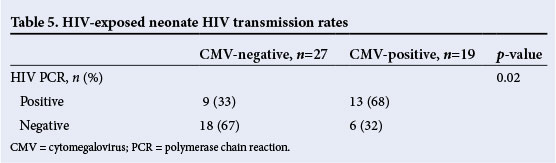
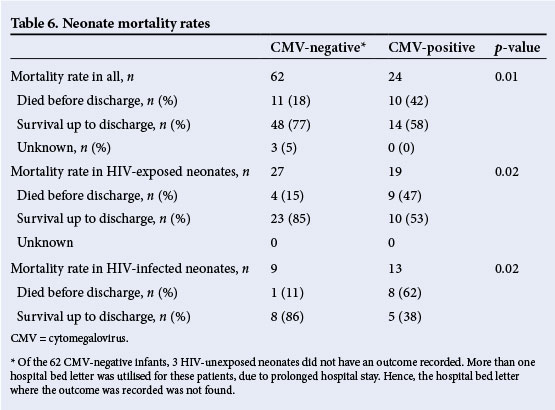
Mortality
The neonates who had congenital CMV were more likely to die (mortality rate 42%) than the CMV-negative (mortality rate 18%) (p=0.01), especially if they were HIV exposed (47% v. 15%,p=0.02) or HIV infected (62% v. 11%, p=0.02) (Table 6).
Discussion
CMV is the most common congenital infection worldwide. However, signs and symptoms are present in only 10% of cases, with up to 90% of these symptomatic congenital CMV-infected neonates developing neurosensory and/or neuromotor impairment.[4] In this single-centre study, hospital records of neonates with suspected and investigated congenital CMV infection were retrospectively reviewed. Comparisons between CMV-infected and -uninfected were made. The presence of thrombocytopenia was the most common reason for suspecting congenital CMV infection. Findings of significance included the tendency for the symptomatic congenital CMV-infected neonates to be HIV exposed and/or HIV-infected, and hepato/splenomegaly being the dominant finding on clinical examination. Neonates who were CMV infected had much lower platelet counts compared with the CMV uninfected. Mortality before hospital discharge was higher in the CMV-infected group, HIV-infected and male neonates. The incidence in this 5-year study period was 0.026%, which is much lower than that reported by Schoub et al.,[5] where they tested 2 250 asymptomatic neonates and reported a prevalence of 0.13% (95% CI 0.5 - 0.39). The reason for the difference in prevalence between the two studies is most likely two-fold. Firstly, Schoub et al.[5] used serology to diagnose CMV infection, whereas in this study we used a shell vial culture and/or pp65. CMV serology tends to give false-positive and false-negative results. Secondly, Schoub et al.[5] tested all babies born to mothers with reactivated CMV infection during their pregnancy, while in the current study only symptomatic newborns were tested irrespective of time of acquisition of maternal CMV infection. Kenneson and Cannon6 performed a systemic review to determine worldwide birth prevalence of symptomatic congenital CMV infection. They found an overall birth prevalence of 0.07% (95% CI 0.03 - 0.56), which is similar to the current study.
In this retrospective review, the most common indication for CMV testing in neonates within the first 3 weeks of life was either the clinical presence of hepato/splenomegaly or evidence of thrombocytopenia. Comparative analysis confirmed a significant association between the presence of severe thrombocytopenia or hepato/splenomegaly, and congenital CMV infection. In the study by Boppana et al.,[7] thrombocytopenia and hepato/splenomegaly was present in 76% and 60% of their congenital CMV-infected cohort, respectively. In the American cohort of Kylat et al.,[8]thrombocytopenia and hepato/splenomegaly were reported as 40 - 45% and 90% prevalent, respectively.
During the study period, the prevalence of HIV infection in mothers attending an antenatal clinic in Gauteng was 30.4%.[9] This record review was done during the era when mother-to-child transmission of HIV was prevented by intrapartum single-dose nevirapine (NVP) to the HIV-infected mother followed by a single oral dose of NVP syrup to the HIV-exposed neonate at birth. Subsequent HIV testing in the neonate was performed at the chronological age of 6 weeks. In the study by Gray et al.,[10] where neonates were randomised within 24 h of delivery to receive either a single oral dose of NVP or zidovudine, the authors reported an HIV transmission rate of 11.9% in the NVP arm of their cohort (tested prior to 6 weeks of age). This is lower than the transmission rate in the current study of 33% in the CMV-uninfected and 68% in the congenital CMV-infected infants. The infants in this study were born with signs or laboratory findings that suggested that they might have acquired HIV infection in utero. Testing most infants at 6 weeks could have led to exaggeration of perinatal or postnatal transmission rate.
Guibert et al.[1] reported on a multicentre cohort of HIV-exposed neonates who showed a higher prevalence of congenital CMV infection in those neonates who had been HIV exposed and subsequently become HIV infected (67% v. 42%; p<0.001). The study did not include a control population to assess the transmission of in utero CMV infection in neonates born to HIV-uninfected mothers. Also, there was no comment on the association of congenital CMV infection in HIV-exposed neonates who were subsequently deemed HIV-negative at 6 weeks of age.[1] Slyker et al.[11]compared a cohort of 51 Kenyan HIV-exposed infants with a control group that was HIV unexposed and therefore HIV uninfected (n=13). The authors reported congenital CMV in 29% and 2.7% of HIV-exposed and -infected, and HIV-exposed and -uninfected neonates, respectively. All neonates were congenital CMV-negative in the control group.[11] Their results suggested that infants born to HIV-positive mothers are more likely to acquire CMV infection than in those born to HIV-negative mothers, especially if they are also HIV infected.
Our study showed the outcome of mortality before hospital discharge to be significantly higher in the congenital CMV-infected subgroup of neonates (42% v. 16%; p=0.01). The major independent poor prognostic factor for the adverse short-term outcome of death before hospital discharge was male gender (p=0.004); male neonates were 23 times more likely to die before hospital discharge (p=0.04). This compares with studies by Boppana et al.,[7] who reported a mortality rate of <5% in the symptomatic CMV-infected neonates, and Kylat et al.,[8] who reported a mortality rate of 7% in their cohort, with significant risk of adverse outcomes if there was abnormal brainstem evoked response audiometry (BAER) (odds ratio (OR) 8.7), head ultrasound (OR 8.5) or brain computed tomography scan (OR 21.0) at presentation. The presence of female gender, and abnormal abdominal or cerebral findings was reported by Maruyama et al.[12] as predictors of adverse outcome. Bristow et al.[13] reported mortality in 41% of neonates while Townsend et al.[14] reported a mortality rate of 10.5% in congenital CMV-infected neonates. The difference in the mortality rate between our study and other studies is that 54% of patients in this study were HIV infected and 38% had very low birth weight. These HIV-infected neonates could have been infected in utero, which could translate to higher mortality rates.[15] In addition, the high mortality rate in our study could be related to delays in initiating antiretroviral treatment.
One of the main strengths of this study was the high number of neonates with symptomatic congenital CMV infection from a single centre. The main limitations of this study include the retrospective nature of the study and the inability to retrieve all the patient records of the congenital CMV-negative neonates. The HIV-exposed neonates were only tested for HIV infection at 6 weeks of life. These HIV-exposed, HIV-infected neonates may have acquired HIV prior to 6 weeks of life.
Conclusion
Neonates presenting with hepato/splenomegaly and thrombocytopenia at birth, and born to HIV-positive mothers, should be investigated for both CMV and HIV infection. HIV-exposed neonates presenting with hepato/splenomegaly and thrombocytopenia at birth should have an HIV-PCR done within the first 48 h of life in order to determine the time of acquisition of HIV infection and enable early initiation of antiretroviral treatment, which is most likely to reduce the mortality observed in this group of patients.
Acknowledgements .Thanks to the NICD for providing urine cultures and pp65 results. A special thanks to Mrs Boitumelo Nhlapo and Mrs Neo Ndlovu for assisting in recovering patients' hospital bed letters and Dr Priyesh Hira for assistance with statistics.
References
1. Guibert G, Warszawski J, le Chenadec J, et al. Decreased risk of congenital cytomegalovirus infection in children born to HIV-1-infected mothers in the era of highly active antiretroviral therapy. Clin Infect Dis 2009;48(11):1516-1525. [http://dx.doi.org/10.1086/598934] [ Links ]
2. Adhikari M, Kauchali S, Moodley S. Clinical profile and morbidity pattern of infants born to HIV-infected mothers in urban South Africa. Indian Pediatr 2006;43:804-808. [ Links ]
3. Martin RJ, Fanaroff AA, Walsh MC. Neonatal-perinatal medicine: Diseases of the fetus and infant, Volume 2. 8th ed. Philadelphia: Mosby Elsivier, 2006:1804,1810. [ Links ]
4. Malm G, Engman ML. Congenital cytomegalovirus infections. Semin Fetal Neonatal Med 2007;12(3):154-159. [http://dx.doi.org/10.1016/j.siny.2007.01.012] [ Links ]
5. Schoub BD, Johnson S, McAnerney JM, et al. Is antenatal screening for rubella and cytomegalovirus justified? S Afr Med J 1993;83(2):108-110. [ Links ]
6. Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007;17(4):253-257. [http://dx.doi.org/10.1002/rmv.535] [ Links ]
7. Boppana S, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: Neonatal morbidity and mortality. Pediatr Infect Dis J 1992;11(2):93-99. [ Links ]
8. Kylat RI, Kelly EN, Ford-Jones EL. Clinical findings and adverse outcome in neonates with symptomatic congenital cytomegalovirus (SCCMV) infection. Eur J Pediatr 2006;165(11):773-778. [http://dx.doi.org/10.1007/s00431-006-0172-6] [ Links ]
9. Goga A, Dinh TH, Jackson D for the South African Prevention of Mother-to-child-transmission Evaluation (SAPMTCTE) Study Group. Evaluation of the effectiveness of the national prevention of mother-to-child transmission (PMTCT) programme at six weeks postpartum in South Africa, 2010. South African Medical Research Council, National Department of Health of South Africa and PEPFAR/US Centers for Disease Control and Prevention. 2012. http://www.doh.gov.za/docs/reports/2012/pmtcteffectiveness.pdf (accessed 1 Oct 2012). [ Links ]
10. Gray GE, Urban M, Chersich MF, et al. A randomized trial of two postexposure prophylaxis regimens to reduce mother-to-child HIV-1 transmission in infants of untreated mothers. AIDS 2005;19(12):1289-1297. [ Links ]
11. Slyker JA, Lohman-Payne BL, John-Stewart GC, et al. Acute cytomegalovirus infection in Kenyan HIV-infected infants. AIDS 2009;23(16):2173-2181. [http://dx.doi.org/10.1097/QAD.0b013e32833016e8] [ Links ]
12. Maruyama Y, Sameshima H, Kamitomo M, et al. Fetal manifestations and poor outcomes of congenital cytomegalovirus infections: Possible candidates for intrauterine antiviral treatments. J Obstet Gynaecol Res 2007;33(5):619-623. [http://dx.doi.org/10.1111/j.1447-0756.2007.00621.x] [ Links ]
13. Bristow BN, O'Keefe KA, Shafir SC, Frank J. Sorvillo FJ. Congenital cytomegalovirus mortality in the United States, 1990 - 2006. PLoS Negl Trop Dis 2011;5(4):e1140. [http://dx.doi.org/10.1371/journal.pntd.0001140] [ Links ]
14. Townsend CL, Peckham CS, Tookey PA. Surveillance of congenital cytomegalovirus in the UK and Ireland. Arch Dis Child Fetal Neonatal Ed 2011;96(6):F398-F403. [http://dx.doi.org/10.1136/adc.2010.199901] [ Links ]
15. Chandwani S, Aditya K, Bebenrot D, et al. Cytomegalovirus infection in human immunodeficiency virus type-1 infected children. Pediatr Infect Dis J 1996;15(4):310-314. [ Links ]
 Correspondence:
Correspondence:
H A Diar
doc.diar@yahoo.com














