Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.8 n.3 Pretoria Sep. 2014
RESEARCH
Hypoglycaemia in children aged 1 month to 10 years admitted to the Children's Emergency Centre of Lagos University Teaching Hospital, Nigeria
E E OyenusiI; A O OduwoleII; O O OladipoIII; O F NjokanmaIV; C I EsezoborV
IMBBS, FMCPaed, MWACP; Department of Paediatrics, Lagos University Teaching Hospital, Idi-Araba, Lagos, Nigeria
IIMBBS, FWACP; Department of Paediatrics, Lagos University Teaching Hospital, Idi-Araba, Lagos, Nigeria
IIIMBBS, MSc, FWACP, FMCPath, DABCC; Staten Island University Hospital, Staten Island, New York, USA
IVMBBS, FMCPaed, FWACP; Department of Paediatrics, Lagos State University Teaching Hospital, Ikeja, Lagos, Nigeria
VMBBS, MPH, FMCPaed, FWACP; Department of Paediatrics, Lagos University Teaching Hospital, Idi-Araba, Lagos, Nigeria
ABSTRACT
BACKGROUND: Hypoglycaemia occurs in many disease states common in the tropics, and may also complicate treatment of malaria. It may contribute significantly to morbidity and mortality.
OBJECTIVES: To determine the prevalence of and clinical conditions associated with hypoglycaemia.
METHODS: A total of 430 patients aged 1 month to 10 years were recruited consecutively from the Children's Emergency Centre of Lagos University Teaching Hospital. Clinical and demographic data were entered into a predesigned study proforma. Blood glucose was determined in the laboratory using the glucose oxidase method. Hypoglycaemia was defined as plasma glucose <2.5 mmol/L.
RESULTS: The median age of the study subjects was 24 months, with a range of 1.5 - 120 months. A total of 248 patients (57.6%) were <24 months old. The mean (standard deviation) blood glucose of all the study subjects was 5.19 (2.05) mmol/L (median 4.9 mmol/L). Twenty-four patients (5.6%) were hypoglycaemic. The predominant disease conditions in which hypoglycaemia occurred were severe malaria, multisystemic infections, marasmus, malignancies and gastroenteritis. Mortality was higher in hypoglycaemic patients than in those without hypoglycaemia (33.3% v. 5.4%, p<0.01).
CONCLUSION: Hypoglycaemia complicates many common childhood illnesses seen in the emergency room and is associated with significant mortality. Hypoglycaemia should be suspected in severely ill children with severe malaria, multisystemic infections, marasmus, malignancies and gastroenteritis.
Hypoglycaemia in children is defined as a whole blood glucose concentration of <2.2 mmol/L or plasma glucose <2.5 mmol/L.[1] It is found in many disease states common in the tropics, such as severe malaria, severe malnutrition, diarrhoea and septicaemia.[2,3] Hypoglycaemia may also complicate treatment with some drugs, such as quinine, used in the treatment of severe malaria,[4] and is associated with significant morbidity and mortality.[2,3,5] Survivors of prolonged hypoglycaemia are prone to neurological complications such as mental retardation, recurrent seizure activity, transient cognitive impairment and neurological deficits.[6]
The absence of clinical symptoms does not always indicate that the glucose concentration is normal or that it has not fallen below optimal level for maintaining brain metabolism.[7] Therefore, in sick children presenting to the emergency room, reliance on clinical manifestations to detect hypoglycaemia may result in missed diagnosis with attendant increase in the risk of complications of hypoglycaemia.
There is a paucity of published data on hypoglycaemia in Nigerian emergency rooms, indicating that not enough attention has been given to the subject in our environment. Hence, this study was designed to determine the prevalence and clinical conditions associated with hypoglycaemia in children admitted to the emergency room in Nigeria.
Methods
This cross-sectional study was conducted at the Children's Emergency Centre of the Lagos University Teaching Hospital (LUTH), Nigeria. All consecutively admitted children, aged 1 month to 10 years, between September 2009 and May 2010 were recruited. Patients were excluded if they were confirmed to have diabetes mellitus or have had glucose-containing intravenous fluids <6 hours before admission. Approval was obtained from the Human Research and Ethics Committee of LUTH before commencement of the study. Written informed consent was also obtained from the parents or caregivers.
Data collection
For each subject recruited, the demographic data, presenting complaints, duration and other relevant information were recorded. On admission to the emergency room, before commencement of fluid therapy, blood glucose was determined using 1.5 mL of blood withdrawn from a convenient peripheral vein, which was put in a fluoride oxalate-containing bottle for standard laboratory analysis using the glucose oxidase method. Hypoglycaemia was defined as laboratory plasma glucose <2.5 mmol/L. Treatment of hypoglycaemia consisted of an immediate intravenous bolus of 0.4 g/kg body weight (BW) of glucose over a minute followed by a continuous dextrose infusion at 8 mg/kg BW/min.[7]
Data management and analysis
The data were analysed using SPSS version 17. Statistical significance was taken at p<0.05. Nutritional status was classified using the WHO Global Database on Child Growth and Malnutrition[8]
Results
A total of 461 children aged 1.5 - 120 months were recruited, but 31 laboratory blood samples were excluded due to improper handling, leaving 430 patients. There were 252 (58.6%) males and 178 (41.4%) females (Table 1). More than half of the entire population (57.6%) comprised children aged <24 months. The overall mean (standard deviation (SD)) age was 38.76 (38.20) months (range 1.5 - 120 months).
Of the 92 (21.4%) patients who were undernourished, 35 (38.0%) had severe undernutrition while 57 (62.0%) had moderate undernutrition. A greater percentage of males than females had a weight-for-age z-score (WAZ) <-2 (23.0% v. 19.1%, p=0.33).
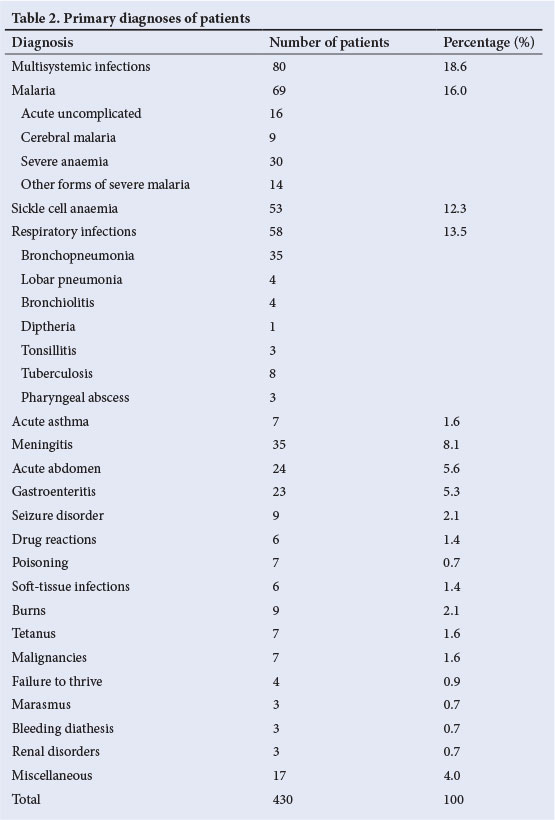
The various diagnoses of the study subjects are shown in Table 2. The most common four conditions (multisystemic infections, malaria, sickle cell anaemia and respiratory infections) accounted for more than half of the diagnoses. Severe anaemia was the most common presentation of severe malaria.
Hypoglycaemic patients
Of the 430 study subjects, 24 (5.6%) were hypoglycaemic (Table 3). A greater percentage of females (7.3%) were hypoglycaemic compared with males (4.4%). The hypoglycaemic patients were fairly distributed between the infancy age group and the under-fives. The prevalence of hypoglycaemia in patients with a WAZ <-2 was almost twice that of children with higher z-scores (p=0.14). An interval between the last meal and sampling of >12 hours was associated with a significantly greater frequency of hypoglycaemia (p<0.01). The mean (SD) duration of time interval after the last meal in hypoglycaemic patients was significantly higher than it was for non-hypoglycaemic patients (9.6 (7.8) hours v. 3.8 (5.3) hours; t=3.59, p<0.05).
The leading diagnoses associated with hypoglycaemia were cerebral malaria and multisystemic infections (Table 4). Other conditions included marasmus, malignancies, gastroenteritis and renal disorders.
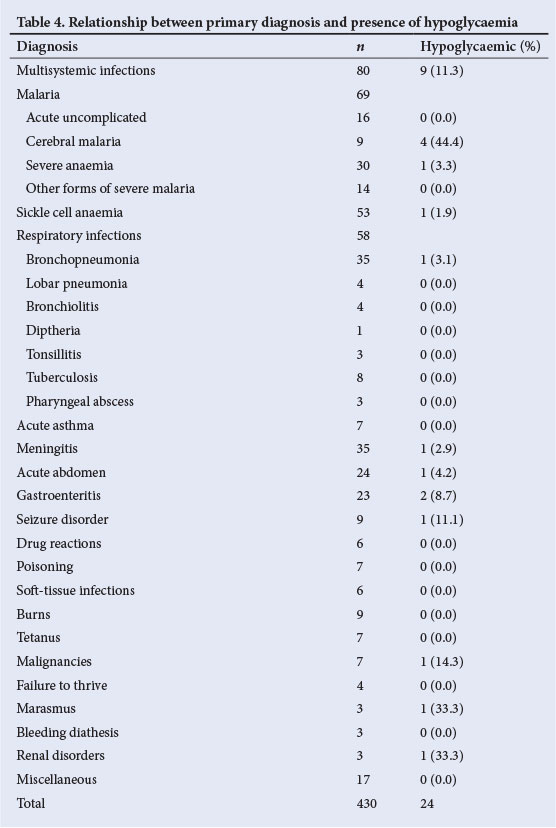
Thirty patients died, resulting in a mortality rate of 7%. A significantly greater percentage (33.3%) of hypoglycaemic patients died in comparison with only 5.4% of the non-hypoglycaemic patients (p=0.000). Hypoglycaemia was associated with mortality in multisystemic infections, cerebral malaria, bronchopneumonia and malignancies (Table 5). There was significant mortality when hypoglycaemia occurred in patients with multisystemic infections (p<0.01). Overall, the mortality rate associated with the various disease conditions in the presence of hypoglycaemia was significantly higher than the mortality rate in the patients who did not have hypoglycaemia (p<0.05). The highest three case fatality rates were associated with malignancies (57.1%), tetanus (28.6%) and cerebral malaria (22.2%).
Discussion
The prevalence of hypoglycaemia in the current study was 5.6%. This is comparable with the rates of 6.4 - 7.3% documented by earlier studies both in Nigeria[2] and outside the country.[3,4] It is, however, much higher than the rate of 0.007% (6.54/100 000 visits) reported in Alabama.[9] Differences in prevalence rate may be explainable on the basis of patient characteristics. In contrast, the observed prevalence rate of 5.6% in the current study is lower than the rate of 18.6% reported in California.[10] This could be due to the higher cut-off definition of hypoglycaemia used in the Californian study, namely plasma glucose <65 mg/dL (3.6 mmol/L)[10] and the group of patients studied, who were in the paediatric intensive care unit.
There was an inverse, though not significant, relationship between age and the prevalence of hypoglycaemia, comparable with a previous Nigerian study by Elusiyan et al.,[2] in which the highest prevalence of hypoglycaemia occurred in children aged 1 - 3 years, and a Kenyan study by Osier et al.[,3] in which the highest prevalence of hypoglycaemia occurred in children aged 2 -4 years. Predominance of hypoglycaemia in the under-fives age group is understandable, considering the peculiar glucose metabolism in this age group. Glucose homeostasis is limited in younger children compared with older children and adults because of their smaller reserves of liver glycogen and muscle protein, coupled with their relatively higher rates of glucose utilisation due to their larger brain-to-body mass ratio.[7] The reduced yield of glucose from glycogenolysis and gluconeogenesis during periods of increased metabolic demand and poor intake associated with illness predispose these patients to hypoglycaemia.
There was a greater proportion of hypoglycaemic patients who had fed >12 hours before presentation, compared with the non-hypoglycaemic patients as has been documented by other African authors.[2,3,11,12] Intervals between the last feed and blood sampling >12 hours were significantly associated with a higher prevalence of hypoglycaemia. This finding is not surprising because inadequate fasting adaptation is a major cause of hypoglycaemia in children, as glycogen reserves are used up after 12 hours without food.[7] This is further worsened by the defective gluconeogenesis in most childhood illnesses.
The different diagnoses in patients with hypoglycaemia observed in the current study consisted of common childhood diseases, similar to the range of diseases reported by earlier studies in Nigeria[2] and other African countries.[3] The leading diagnosis associated with hypoglycaemia in the current study was severe malaria. This is comparable with observations in other African studies,[2,3] and the prevalence rate of hypoglycaemia in cases of severe malaria is comparable with that in a Kenyan study. [13] However, this rate was considerably higher than what was reported in an earlier Nigerian study[2] and much lower than that reported from The Gambia.[4] These wide variations in rates reported may be related to the type of severe malaria and the overall severity of illnesses in the various studies.
In the current study, nearly half of the patients with cerebral malaria had hypoglycaemia. This prevalence is almost twice the rates of 17% and 20% recorded by other African authors, respectively. [11,12] The difference in prevalence rates may be attributable to a difference in severity of the illnesses seen in the various studies. Considering the potentially serious sequelae of cerebral malaria complicated by hypoglycaemia, a high index of suspicion is essential in order to facilitate adequate treatment.
The prevalence rate of gastroenteritis in the current study is higher than that reported in a study in Bangladesh[5] A possible explanation for the difference in prevalence rates could be because all consecutive patients in the current study were tested for hypoglycaemia, while in the Bangladesh study, only patients with altered consciousness were tested for hypoglycaemia in the 2nd 4 months of the study period. However, the observed prevalence rate in the present study is lower than the rate reported in another diarrhoea-based study in India.[14] The smaller sample size of 10 in the Indian study could have contributed to the apparently higher prevalence rate. Fasting, malnutrition, ketosis and impairment of gluconeogenesis have been suggested as the mechanisms of hypoglycaemia in diarrhoeal diseases.[5,15]
Hypoglycaemia was also prevalent in patients with bacterial infections involving multiple systems in the present study. The prevalence rate is comparable with that reported in another Nigerian study. [2] Hypoglycaemia in infective processes has been attributed to increased peripheral glucose utilisation, which may be caused by fever, hypotension or decreased tissue perfusion,[61] while some authors[15] implicated inhibition of gluconeogenesis. In addition, endotoxins produced by micro-organisms in infectious processes have been observed to stimulate increased insulin secretion, contributing directly to depletion of hepatic glycogen stores[15]
In the current study, the prevalence of hypoglycaemia in patients with moderate to severe undernutrition was almost twice that of children without undernutrition. Specifically, one out of three patients (33%) with marasmus had hypoglycaemia. This is close to the prevalence rate observed by Elusiyan et al,[2] but it must be pointed out that the number of subjects involved was very small and interpretation probably anecdotal. This observation is important considering that other studies in Kenya[3] and Mozambique, [12] with considerably more severely undernourished subjects, reported lower prevalence rates of hypoglycaemia. The fatty infiltration of the liver as occurs in severe undernutrition causes glycogen and gluconeogenic substrate depletion. Furthermore, the defect in glycogenolytic pathways and limited lipolysis also contribute to the occurrence of hypoglycaemia in undernutrition. [18]
There was a significant association between hypoglycaemia and increased mortality in the current study. A similar association had been reported in several other studies.[2,3,10,12,18] It is obvious that hypoglycaemia is in some way linked to mortality. The major diagnoses associated with high mortality in patients with hypoglycaemia in the present study included multisystemic infections, cerebral malaria, bronchopneumonia and malignancies. This is in agreement with other studies,[2,12] which identified cerebral malaria and infections as major diagnoses associated with mortality in hypoglycaemic patients. However, these diseases are severe conditions that, on their own, can be fatal.
Conclusion
Hypoglycaemia caused complications in common childhood diseases in the Children's Emergency Centre of LUTH. Ill children aged <5 years and those with low WAZs were particularly susceptible to hypoglycaemia. Severe malaria, multisystemic infections, marasmus, malignancies and gastroenteritis were the predominant diagnoses associated with hypoglycaemia. Prolonged interval (>12 hours) between the last meal and time of presentation was significantly associated with the occurrence of hypoglycaemia. It is therefore recommended that blood glucose should be measured in all children ill enough to be admitted to the emergency room. A follow-up study of the surviving hypoglycaemic patients will be useful to screen for any associated long-term sequelae.
References
1. Cornblath M, Schwartz R. Disorders of carbohydrate metabolism in infancy. 3rd ed. Boston: Blackwell Scientific Publications, 1991:1-53. [ Links ]
2. Elusiyan JBE, Adejuyigbe EA, Adeodu OO. Hypoglycaemia in a Nigerian paediatric emergency ward. J Trop Pediatr 2006;52(2):96-102. [http://dx.doi.org/10.1093/tropej/fmi068] [ Links ]
3. Osier FHA, Berkley JA, Ross A, Sanderson F, Mohammed S, Newton CRJC. Abnormal glucose concentrations on admission to a rural Kenyan district hospital: Prevalence and outcome. Arch Dis Child 2003;88(7):621-625. [ Links ]
4. White NJ, Warrel DA, Chanthavanich P, et al. Severe hypoglycaemia and hyperinsulinaemia in falciparum malaria. N Engl J Med 1983;309(2):61-63. [ Links ]
5. Bennish ML, Azab AK, Rahman O, Phillips RE. Hypoglycaemia during diarrhoea in childhood: Prevalence, pathophysiology and outcome. N Engl J Med 1990;322(19):1357-1363. [ Links ]
6. Idro R, Carter JA, Fegan G, Neville BGR, Newton CRJC. Risk factors for persisting neurological and cognitive impairments following cerebral malaria. Arch Dis Child 2006;91(2):142-8. [http://dx.doi.org/10.1136/adc.2005.077784] [ Links ]
7. Thornton TS, Finegold DN, Stanley CA, Sperling MA. Hypoglycaemia in the infant and child. In: Sperling MA, ed. Paediatric Endocrinology. 2nd ed. Philadelphia: Saunders, 2002:135-159. [ Links ]
8. World Health Organization. Global Database on Child Growth and Malnutrition. www.who.int/nutrition/topics/malnutrition/en/index.html (accessed 20 July 2011). [ Links ]
9. Pershad J, Monroe K, Atchison J. Childhood hypoglycaemia in an urban emergency department: Epidemiology and a diagnostic approach to the problem. Paediatr Emerg Care 1998;14(4):268-271. [ Links ]
10. Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia and glucose variability with morbidity and deaths in the pediatric intensive care unit. Pediatrics 2006;118(1):173-179. [http://dx.doi.org/10.1542/peds.2005-1819] [ Links ]
11. Nwosu SU, Lesi FEA, Mafe AG, Egri-Okwaji MTC. Hypoglycaemia in children with cerebral malaria seen at the Lagos University Teaching Hospital: Predisposing factors. Nig Med J 2004;45(1):9-13. [ Links ]
12. Solomon T, Felix TM, Samuel M, et al. Hypoglycaemia in paediatric admissions in Mozambique. Lancet 1994;343(8890):149-150. [ Links ]
13. English M, Wale S, Binns G, Mwangi I, Sauerwein H, Marsh K. Hypoglycaemia on and after admission in Kenyan children with severe malaria. QJM 1988;91(3):191-197. [ Links ]
14. Bhattacharya SK, Bhattacharya MK, Dutta D, et al. Vibrio cholerae 0139 in Calcutta. Arch Dis Child 1994;71:161-162. [ Links ]
15. Filkins JP, Cornell RP. Depression of hepatic gluconeogenesis and the hypoglycaemia of endotoxic shock. Am J Physiol 1974;227(4):778-781. [ Links ]
16. Miller SI, Wallace RJ Jr, Musher DM, Septimus EJ, Kohl S, Baughn RE. Hypoglycaemia as a manifestation of sepsis. Am J Med 1980;68(5):649-654. [ Links ]
17. Yelich MR, Filkins JP. Mechanism of hyperinsulinaemia in endotoxicosis. Am J Physiol 1980;239(2):E156-161. [ Links ]
18. White NJ, Miller KD, Marsh K, et al. Hypoglycaemia in African children with severe malaria. Lancet 1987;1(8535):708-711. [ Links ]
 Correspondence:
Correspondence:
E E Oyenusi
(ebikike@yahoo.com)














