Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.8 n.3 Pretoria Sep. 2014
RESEARCH
Comparative weight gain with ready-to-use therapeutic food in stunted HIV-infected and -uninfected children in a Nigerian Hospital
O K IgeI; R E OladokunII; O KikelomoIII
IMBBS, MSc, FWACP; Department of Community Medicine, University College Hospital, Ibadan, Nigeria
IMBBS, MPH, FMCPaed; Department of Community Medicine, University College Hospital, Ibadan, Nigeria
IIIMBBS, FMCPaed, FWACP; Department of Paediatrics, College of Medicine, University of Ibadan and University College Hospital, Ibadan, Nigeria
ABSTRACT
OBJECTIVE: To bridge the management gap between nutritional rehabilitation for severe acute malnutrition (SAM) and chronic malnutrition, this study investigated to what extent ready-to-use therapeutic food (RUTF) promotes growth in children with long-term nutrition deficit with superimposed SAM.
METHODS: A total of 225 (164 HIV-negative and 61 HIV-positive) chronically malnourished children (aged 6 - 60 months) with superimposed SAM were enrolled. Children were provided 92 g packets of an RUTF, Plumpy'Nut, based on an estimated requirement of 200 kcal/kg body weight (BW)/day. Children were fed Plumpy'Nut over a 2-week period, and weight was assessed weekly. Weight gain was compared for HIV-positive children and HIV-negative children.
RESULTS: On day 15, the HIV-positive group had a median weight gain of 645 g compared with 670 g in the HIV-negative group (difference 25 g, p=0.784). Similarly, rate of weight gain per kilogram BW per day was comparable for both groups of children (13.2 g/kg BW per day for HIV-negative children v. 11.9 g/kg BW per day for HIV-positive children, p=0.353). On day 15, the proportions of HIV-positive and HIV-negative children who had sustained weight gain were not significantly different.
CONCLUSION: Chronically malnourished children with superimposed SAM benefit from the use of RUTF as much as children without chronic nutritional deprivation, regardless of HIV status.
In Africa, children who suffer from chronic malnutrition are more likely to die as a result of a common childhood disease than those who are adequately nourished. Even though the harmful effects of malnutrition can be reversed, if the opportunity is missed, the child may never make up the difference in growth and development, and will be adversely affected for the rest of his or her life.[1] In many instances, wasting and stunting coexist within population groups, yet the management of severe acute malnutrition (SAM) is often isolated from that of chronic malnutrion.[2] In response to recommendations for integrated management of malnutrition,[2] it becomes important to evaluate therapeutic responses to nutritional rehabilitation for acutely malnourished children who are also often chronically malnourished.
For several years, the effectiveness of ready-to-use therapeutic food (RUTF) for the treatment of severe malnutrition has been publicised for use in severely malnourished children.[3] The effectiveness of RUTF in nutrition rehabilitation has also been demonstrated for both HIV-positive and HIV-negative children.[4] Many of these studies have investigated RUTF in SAM cases only. In an attempt to bridge the management gap between nutritional rehabilitation for SAM and chronic malnutrition, this study investigated to what extent RUTF promotes weight gain in children with long-term nutritional deficit with superimposed SAM. The effectiveness of RUTF in producing weight gain is compared for HIV-positive children and HIV-negative children.
Methods
All severely malnourished children referred for nutrition rehabilitation at the Pediatric Unit of the University College Hospital, Ibadan, Nigeria, during a 1-year period (2011 - 2012) were recruited into the study. The hospital is a national referral and teaching hospital located in South-west Nigeria, but patients come from all over the country. The main inclusion criterion was stunting, defined as a height-for-age indicator z-score of <-3 in addition to a weight-for-height z-score (WHZ) of <-3 according to the World Health Organization (WHO) references.[5] All z-scores were derived using the WHO Anthro software version 3.1.[6] Children were eligible if they had been hospitalised due to complications of SAM (septicaemia, pneumonia, gastroenteritis, tuberculosis, measles or electrolyte imbalance) or if they were referred from the outpatient department for treatment of malnutrition with either oedema or a WHZ of <-3. The mothers or caregivers of the participants gave their informed consent before a child was enrolled.
The ages of the children were between 6 and 60 months. The following parameters were recorded for all the children: demographic characteristics (age and sex), clinical features (weight, height/length and presence of oedema), diarrhoea, appetite, vomiting, fever and respiratory distress. The children were divided into HIV-positive and HIV-negative groups. HIV testing was by rapid antibody tests, and confirmation for children <18 months old was by DNA polymerase chain reaction (PCR). For those >18 months old, Western blot was used to confirm HIV infection.
In this study, a total of 225 chronically malnourished children with superimposed SAM, with and without complications, were included. Minimum sample size was estimated for a power of 80%, p<0.05 for a two-sided t-test, and an assumed mean (standard deviation (SD)) weight gain after 14 days of 353 (365) g.[4] This resulted in a minimum sample size of 24 participants per study group (StatsDirect, UK). However, we worked with the total number of children who presented within the recruitment period and who met the inclusion criteria, namely 164 HIV-negative and 61 HIV-positive children.
The intervention was an RUTF brand called Plumpy'Nut (Nutriset, France), an energy-dense lipid paste made of peanut butter, milk powder, oil, sugar, minerals, vitamins and protein mix.[7] Children were provided 92 g packets of Plumpy'Nut, containing 500 kcal per packet, according to body weight (BW), based on an estimated average requirement of 200 kcal/kg BW/day. The children were fed Plumpy'Nut over a 2-week period starting from enrolment. Weight was assessed weekly. All children continued their regular treatment for any other comorbid conditions.
BW was measured to the nearest 100 g using calibrated analogue scales at baseline and at the end of each week. Height was measured to the nearest 1 mm using a portable stadiometer. Length was also measured for younger children to the nearest 1 mm using a calibrated board on which children were laid supine. The primary end point was the amount of weight gain over a period of 2 weeks (14 days) and sustained weight gain (at two consecutive weekly weighings).
Statistical analysis was performed using SPSS version 16. Medians were used to calculate the central tendency and range for the spread of age, weight and height. To determine differences with regard to the weight change by HIV status, χ2 and Wilcoxon-Mann-Whitney analyses were used. A two-tailed p-value <0.05 was considered significant. Binary logistic regression models were constructed using sustained weight gain as the outcome variable. The appropriate important baseline data of clinical significance was included in the model and used for adjustment.
Results
HIV infection was detected in 61 children (27%). The median age of HIV-positive children was 16 months (range 6 - 60 months), which was not significantly different from the median age of 14 months (range 6 - 60 months) for the HIV-negative children. Neither the baseline weight nor the height was significantly different for the two categories of patients. Most of the clinical parameters were comparable except for the occurrence of fever and diarrhoea, which were both more common in HIV-positive children (p<0.05). The presence of oedematous malnutrition was not affected by HIV status (Table 1).
On day 15, the HIV-positive group had a median weight gain of 645 g compared with 670 g in the HIV-negative group (difference 25 g, p=0.784). Similarly, rate of weight gain per kilogram BW per day was comparable for both groups of children (13.2 g/kg BW/ day for HIV-negative children v. 11.9 g/kg BW/day for HIV-positive children, p=0.353). On day 15, the proportions of HIV-positive and HIV-negative children who had sustained weight gain were not significantly different (Table 2).
There were no significant differences in the proportion of children who gained weight among HIV-positive or -negative children when the inpatients were compared with outpatients (Table 3).
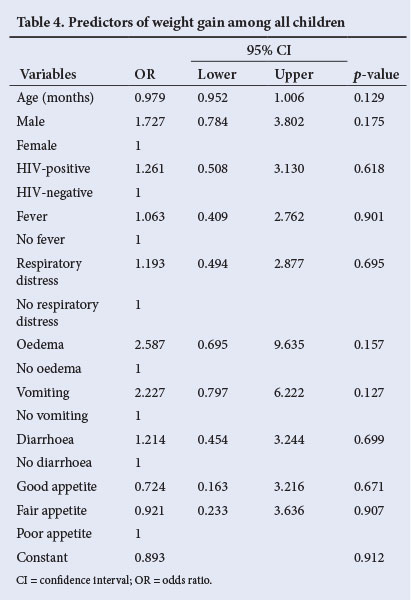
Table 4 shows that on multivariate analysis, none of the baseline clinical or demographic parameters significantly influenced weight gain in this study.
Discussion
This study assessed weight gain following RUTF use for SAM among children who were also stunted. Results indicated that the use of RUTF produced somewhat higher weight gain than the average recorded weight gain in patients with straightforward SAM. As shown in a meta-analysis of published peer-reviewed literature on the use of 'therapeutic nutrition products' such as RUTF in SAM, reported pooled mean weight gain with the use of RUTF was 3.2 g/kg BW/day (95% confidence interval 3.06 - 3.34 g/kg BW/day).[8] This is lower than the results found in this study, namely 13.2 g/kg BW/day for HIV-negative children and 11.9 g/kg BW/day for HIV-positive children. Being chronically malnourished did not appear to limit the therapeutic benefits of nutrition rehabilitation with RUTF. Rates as high as >10 g/kg BW/day such as found in this study have also been reported in other studies.[7] This higher rate of weight gain might be related to the underlying chronic nutritional deprivation; some studies have reported higher mean weight gain in SAM compared with moderately malnourished children.[9]
None of the patient characteristics (HIV status, demography, presence of complications) nor admission status (inpatient/outpatient) significantly influenced weight gain as has been reported by some studies.[10-12] This indicates that the therapeutic benefits of RUTF are not limited by underlying clinical or demographic characteristics.
Study limitations
Certain limitations are recognised in this study. First the period of evaluation was short, and as such other outcomes such as mortality and attainment of recommended weight-for-age could not be assessed. However, since average recovery period has been reported to range from 14 to 21 days,[7,9] it is not expected that this might have affected the results.
Conclusion
The findings from this study demonstrate that chronically malnourished children with superimposed SAM benefit from the use of RUTF, probably even more than children without chronic nutritional deprivation. Even though chronic malnutrition may be irreversible after a particular age,[3] correction of SAM among this group of children should be prioritised, and measures put in place to prevent chronic deprivation. This simple, effective treatment needs to be made available to all children who are affected by acute malnutrition through integrated clinical care programmes linking therapeutic feeding and routine clinical care in both HIV treatment programmes and general paediatric units. Making this therapy more widely available in the developing world where childhood malnutrition is rampant should be a priority.[13]
References
1. Save the Children. Chronic malnutrition: Key questions. http://www.savethechildren.org.uk/about-us/what-we-do/chronic-malnutrition-key-questions (accessed 6 July 2013). [ Links ]
2. Bergeron G, C astleman T. Program responses to acute and chronic malnutrition: Divergences and convergences. Adv Nutr Int Rev J 2012;3(2):242-249. [http://dx.doi.org.10.3945/an.111.001263] [ Links ]
3. USAID Impact. Ready-to-use therapeutic food. http://blog.usaid.gov/2011/10/ready-to-use-therapeutic-food/ (accessed 27 June 2013). [ Links ]
4. World Health Organization. Community-Based Management of Severe Acute Malnutrition. Geneva: World Health Organization, 2007. [ Links ]
5. World Health Organization Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization, 2006. [ Links ]
6. World Health Organization. WHO Anthro 2005 for personal computers manual. http://www.who.int/childgrowth/software/WHOAnthro2005_ PC_Manual.pdf (accessed 20 June 2013). [ Links ]
7. Nutriset. Severe acute malnutrition. http://www.nutriset.fr/en/product-range/severe-acute-malnutrition.html (accessed 27 June 2013). [ Links ]
8. Van der Kam S, Swarthout T, Niragira O, et al. Ready-to-use therapeutic food for catch-up growth in children after an episode of Plasmodium falciparum malaria: An open randomised controlled trial. PLoS ONE 2012;7(4):e35006. [http://dx.doi.org/10.1371/journal.pone.0035006] [ Links ]
9. Gera T. Efficacy and safety of therapeutic nutrition products for home based therapeutic nutrition for severe acute malnutrition a systematic review. Indian Pediatr 2010;47(8):709-718. [ Links ]
10. Hossain MI, Dodd NS, Ahmed T, et al. Experience in managing severe malnutrition in a government tertiary treatment facility in Bangladesh. J Health Popul Nutr 2009;27(1):72-79. [ Links ]
11. Collins S, Dent N, Binns P, Bahwere P, Sadler K, Hallam A. Management of severe acute malnutrition in children. Lancet 2006;368(9551):1992-2000. [http://dx.doi.org/10.1016/S0140-6736(06)69443-9] [ Links ]
12. Bachou H, Tylleskár T, Downing R, Tumwine JK. Severe malnutrition with and without HIV-1 infection in hospitalised children in Kampala, Uganda: Differences in clinical features, haematological findings and CD4+ cell counts. Nutr J 2006;16;5(1):27. [http://dx.doi.org/10.1186/1475-2891-5-27] [ Links ]
13. Sandige H, Ndekha MJ, Briend A, Ashorn P, Manary MJ. Home-based treatment of malnourished Malawian children with locally produced or imported ready-to-use food. J Pediatr Gastroenterol Nutr 2004;39(2):141-146. [ Links ]
 Correspondence:
Correspondence:
O K Ige
(drsimbo@yahoo.co.uk)














