Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.8 n.1 Pretoria Feb. 2014
RESEARCH
Reticulocyte haemoglobin content as a diagnostic tool for iron deficiency and iron-deficiency anaemia in ill infants and children
P D R SwartI; K RautenbachII; J E RaubenheimerIII
IMB ChB; Department of Paediatrics and Child Health, University of the Free State, Bloemfontein, South Africa
IIMB ChB, MMed (Paed), Cert Med Oncol (Paed); Department of Paediatrics and Child Health, University of the Free State, Bloemfontein, South Africa
IIIPhD; Department of Biostatistics, University of the Free State, Bloemfontein, South Africa
ABSTRACT
BACKGROUND: The diagnosis of iron deficiency (ID) and iron-deficiency anaemia (IDA) in ill children is complicated by the unreliability of serum ferritin (S-ferritin). The presence of a microcytic, hypochromic anaemia suggests IDA but is not specific. There is a need for a diagnostic test that will be accessible, cost-effective and accurate for the diagnosis of ID in ill children. Studies done in healthy children have reported that reticulocyte haemoglobin content (CHr) is a reliable diagnostic test for ID, eliminating the need for S-ferritin determination.
OBJECTIVE: To evaluate the accuracy of CHr to diagnose ID and IDA in ill infants and children.
METHODS: A prospective, descriptive study was conducted. One hundred children, aged 6 months to 6 years, who were admitted to Pelonomi Regional Hospital, Bloemfontein, South Africa, during July 2012 and August 2012 were included.
RESULTS: The study group was divided into an iron-deficient group and an iron-sufficient group based on transferrin saturation (TfS). A statistically significant difference was found between mean corpuscular haemoglobin, serum transferrin and CHr in these two groups (p=0.0001). The sensitivity of a CHr level <29 pg to detect ID was 86%, and the specificity was 50%.
CONCLUSION: CHr is an accurate diagnostic test for ID, and for IDA in combination with a low haemoglobin level, in ill infants and children.
Iron deficiency (ID) is one of the main contributing factors of anaemia in infancy and childhood, with an average of 47% of pre-school children and 25% of school-age children affected globally, according to the World Health Organization (WHO). Sufficient iron stores are critical for the increased demands of growth during the first few years of life and during adolescence.[1]
Recent research has indicated that ID has several unwanted neurological sequelae. Not only is it implicated to cause poor social interaction,[2] but it also seems to have a detrimental effect on development[3] and cognitive function.[4] ID has also been linked to sleep alterations,[5] breath-holding spells,[6] cerebral venous sinus thrombosis[7] and idiopathic intracranial hypertension.[8] With prolonged severe ID, the neurological sequelae may eventually become irreversible.[2]
During the National Food Consumption Survey in 2005, it was noted that the proportion of children aged 1 - 9 years who had ID and iron-deficiency anaemia (IDA) in the Free State Province of South Africa (SA) was 18.9% and 11.6%, respectively.[9] In 2010 the prevalence of suspected IDA at Pelonomi Regional Hospital in Bloemfontein was approximately 15%, assuming that a hypochromic, microcytic blood picture indicated IDA.
The causes of ID in childhood are multifactorial with inadequate dietary intake being the most common cause. The high prevalence of protein-energy malnutrition in SA also contributes as a result of impaired iron absorption. Blood loss in infants and childhood is not common, but cow's milk enteropathy and helminth infestations are well-known causes of occult bleeding in this population, and may contribute to IDA.[1]
The gold standard for the diagnosis of ID and IDA is a bone marrow examination to assess iron stores, but this is a painful, invasive and costly procedure. Currently, the best alternative to diagnose ID is a low serum ferritin (S-ferritin) level, indicating low iron stores. Low S-ferritin in combination with a low haemoglobin (Hb) level is used to diagnose IDA.[10]
Being an acute phase reactant, S-ferritin is an unreliable marker of ID in the presence of acute or chronic inflammation or illness. In ill or hospitalised children, the presence of hypochromic, microcytic red blood cells may indicate ID, but this is not specific.[12] Transferrin saturation (TfS) may also be used to assess for ID, but is seldom requested because of financial constraints.[10]
There is a need for a diagnostic test that will be readily accessible, cost-effective and accurate for the diagnosis of ID in ill infants and children. Periods of hospitalisation may be the only opportunity to screen for ID and prevent its neurological sequelae before they become irreversible.
Studies among healthy children from Lithuania, Saudi Arabia and the USA, by Kiudeliene et al.,[11] Bakr and Sarette,[13] and Ullrich et al.,[14] respectively, have reported that the reticulocyte haemoglobin content (CHr) is a reliable diagnostic test for ID, and eliminates the need for iron studies. CHr measures the amount of Hb found in reticulocytes, providing an accurate measure of functional iron available for erythropoiesis over the preceding 3 - 4 days.[10] CHr is not affected by inflammation, malignancy or anaemia of chronic illness. Although not currently used routinely because of poor availability and a lack of international, accredited reference ranges, studies have shown that CHr has potential as a diagnostic tool in ID and IDA in infants and children.[10]
Studies that were performed internationally to assertain whether CHr is an accurate diagnostic test for ID and IDA used healthy infants and children as study subjects. According to our knowledge, no studies have been performed locally or among ill, hospitalised children.[11,13,14]
Objective
The primary objective was to evaluate the accuracy of using CHr to diagnose ID and IDA in ill, hospitalised infants and children.
The secondary objective was to assess the prevalence of ID and IDA in our study population.
Methods
We conducted a prospective, descriptive study. Children were included when they met the following inclusion criteria: (i) aged 6 months - 6 years; (ii) admitted to the paediatric section of Pelonomi Regional Hospital; and (iii) the clinical information, as requested on the data form, was available. Children were excluded if they: (i) had a blood transfusion within the month prior to admission; (ii) were on iron supplementation during the past month; (iii) were known to have thalassaemia or other haematological diseases; (iv) were known to have a chronic illness (e.g. chronic renal failure or liver failure) or chronic infections (e.g. tuberculosis, although children who were HIV-positive without any other comorbidities were included); or (v) were known to have any malignancies. The study budget only allowed for the cost of the laboratory studies of 100 participants. Of the 436 children aged 6 months - 6 years who were admitted to the paediatric section of Pelonomi Regional Hospital during July 2012 and August 2012, 100 (22.94%) were randomly selected to participate in the study.
HIV status was assessed on admission using either: (i) an HIV rapid test if HIV-unexposed or aged >18 months, taking into consideration whether the infant was still breastfeeding; (ii) an HIV polymerase chain reaction (PCR) test in exposed infants aged <18 months; or (iii) a history of HIV infection/receipt of antiretroviral therapy (ART).
Weight and height were assessed on admission and weight-for-age, height-for-age and weight-for-height were plotted using the WHO Z-score growth charts.
Blood samples were collected for a full blood count, CHr and iron studies including S-ferritin, serum transferrin (S-transferrin) and serum iron (S-iron). The TfS value was calculated using the S-ferritin and S-transferrin levels. Special investigations were done by PathCare in Bloemfontein and the results were entered into a set data sheet per study participant.
The study group was divided into an iron-deficient and an iron-sufficient group based on the TfS level: <25% suggested ID and >25% suggested iron sufficiency.[12] These groups were further subdivided into anaemic and non-anaemic groups based on Hb values (Hb <11 g/dl). Since S-ferritin is an acute phase reactant and was expected to be raised in most ill children, this parameter was not used to determine iron status.
Statistical analysis
The analysis was done with assistance from the Department of Biostatistics, University of the Free State. Data from the groups which were identified were compared by the use of either the i-test or Fisher's exact test. Sensitivity and specificity were calculated using a 2x2 table with a DAG-stat Excel spreadsheet[17] A p-value <0.05 was considered to be statistically significant, and where relevant, 95% confidence intervals (CIs) were calculated.
Ethics approval
Approval was granted by the University of the Free State's ethics committee. Informed consent was obtained from the parent/ caregiver before blood sample collection.
Results
Data were available for the 100 included patients. Baseline characteristics are shown in Table 1. The study group had significantly more males (95% CI 52.5 - 70.9) than females. Iron sufficiency/deficiency and anaemia/ non-anaemia divisions were determined as seen in Fig. 1.
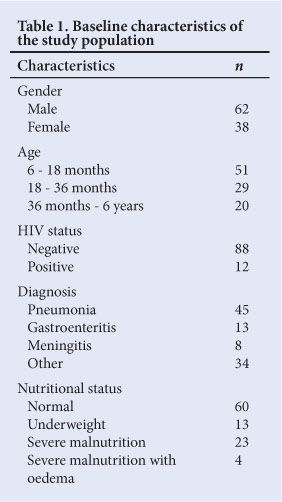
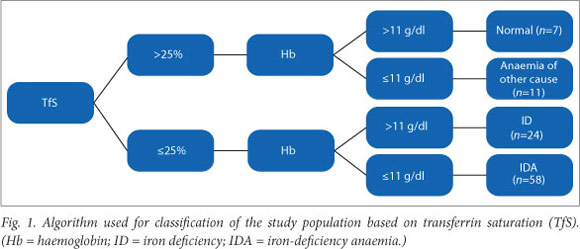
The CHr was assessed in relation to TfS. A CHr value <29 pg was used to indicate the presence of ID.[18] Table 2 indicates the classification of the study population based on CHr. A CHr level <29 pg was significantly useful for the diagnosis of ID and IDA (c2 =21.70; df=3; Fisher's exact p<0.01).
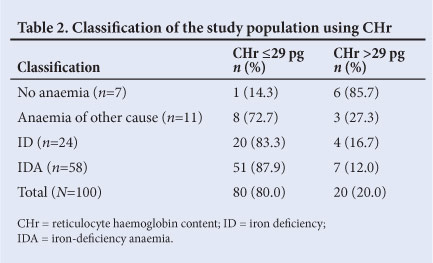
Upon division of the sample into an iron-deficient and iron-sufficient group only (i.e. only on the basis of TfS <25%), the mean CHr values were 24.74% and 28.67%, respectively, revealing a statistically significant difference (t=-4.34; p<0.01) (Table 3).
Red blood cell parameters, S-ferritin and S-transferrin values are presented in Table 4. A statistically significant difference was found between the groups for mean corpuscular haemoglobin (MCH) (p=0.027), S-transferrin (p=0.0002) and CHr (p=0.0001).
The sensitivity of a CHr value <29 pg to detect ID was 86% (95% CI 77.3 - 93.1) and the specificity was 50% (95% CI 26.0 - 74.0).
An unexpected finding was that a group of children who presented with a TfS >25% (i.e. iron-sufficient) had an Hb level <11 g/dl (i.e. anaemic), but a CHr level <29 pg (Table 5). The TfS is calculated by using the S-iron and S-transferrin values; therefore, a low S-transferrin with a normal S-iron level will result in a normal TfS value. The known causes for low S-transferrin include chronic illness, malnutrition and S-transferrin being a negative acute phase protein.[15,16] In five of the eight cases with a normal TfS, anaemia and a low CHr, the S-ferritin level was well above normal, probably due to the current acute illness.
Table 6 shows the haematological and biochemical parameters of the severely malnourished children with oedema. All subjects had a low CHr suggestive of ID. The group also had low to borderline-normal S-transferrin levels, with two patients having very high S-ferritin levels suggestive of acute inflammation.
Table 7 shows the haematological and biochemical parameters of the HIV-positive participants. All subjects had an Hb level <11 g/dl (i.e. anaemic); ten of the 12 (83%) had a TfS <25% (i.e. iron-deficient); and only seven (58.3%) had a CHr level <29 pg. These children had an S-ferritin range of 45 - 1 100 µg/l.
Discussion
ID in childhood not only causes anaemia, but also contributes to neurodevelopmental problems.[2-6] The current practice at Pelonomi Regional Hospital is to treat children with suspected IDA as indicated by haematological parameters, and prescribe iron supplementation upon discharge. On follow-up these haematological parameters are repeated to monitor the response to iron supplementation. The pitfall to this approach is that iron supplementation is only initiated in children in whom anaemia is already present - i.e. microcytic anaemia (mean corpuscular volume (MCV) <73 fl; Hb <11 g/dl). Children are not routinely screened for earlier phases of ID, when anaemia is not yet present. Another problem is that anaemia of chronic illness may also present with a low Hb level and microcytosis, in which case iron supplementation is not indicated. With anaemia of chronic disease, exogenous iron is diverted into the reticulo-endothelial system, which leads to the limited availability of iron for erythropoiesis.[16]
We aimed to evaluate the accuracy of using CHr, a relatively new haematological test in our setting, to diagnose ID in acutely ill children. Reticulocyte production indices are known to be used to assess bone marrow activity, reflecting the balance between iron and erythropoiesis, with CHr serving as an accurate reflection of the available iron for erythropoiesis.[13]
The aforementioned international studies used different reference ranges for CHr: Kiudeliene et al. used <28.55 pg,[11] Bakr and Sarette <26 pg[13] and Ullrich et al. <27.5 pg.[14] Since there is currently no international, accredited reference range for CHr and no reference range available for SA, we focussed on the upper limit of abnormal found in the literature, i.e. CHr <29 pg.[18]
In our study, the group of children who had ID without anaemia (TfS <25% and Hb >11 g/dl) had a mean CHr level of 26.75 pg. This is comparable to the results found by Bakr and Sarette,[13] where the mean CHr for the iron-deficient group was 27.9 pg. The children with IDA in our study (Hb <11 g/dl and TfS <25%) had a mean CHr of 23.91 pg. In Bakr and Sarette's study, the IDA group's mean CHr was 23.1 pg.[13]
Both Kiudeliene et al. and Ullrich et al. compared the iron-deficient group to a normal or control group. In Kiudeliene et al.'s study, the mean CHr for the iron-deficient and control groups was 25.58 pg and 29.25 pg, respectively.[11] In Ullrich et al.'s study, the mean CHr for the iron-deficient and iron-sufficient (control) groups was 26 pg and 28.1 pg, respectively.[14] The results of our study are comparable with these findings: the iron-deficient group (TfS <25%) had a mean CHr of 24.74 pg and the iron-sufficient group (TfS >25%) 28.67 pg (statistically significant difference; p=0.0001).
The results of our study suggest that CHr, with a cut-off value of 29 pg, is an accurate marker for the diagnosis of ID, with a sensitivity of 86%. Kiudeliene et al'.s study, which used a lower cut-off value of 28 pg for CHr, had a sensitivity of 76%,[11] and Ullrich et al'.s study, which used a cut-off of 27.5 pg, had a sensitivity of 83%.[14]
The specificity of using CHr as a diagnostic tool for ID in our study was 50%; that of Kiudeliene et al.'s study was 78.4%,[11] and Ullrich et al.'s study was 72%.[14] A possible explanation for the lower specificity in our study includes the presence of a sub-group of patients in our cohort who had anaemia with a normal TfS level, but a low CHr level, suggestive of anaemia of chronic disease with underlying ID.
As mentioned, our cohort included a group of infants and children who had a normal TfS but a low CHr level. This group may have represented a combination of anaemia of chronic disease and IDA. Another explanation might be that TfS levels were overestimated due to the use of a mathematical formula in their calculation. In clinical practice these patients would be followed up for their anaemia, and haematological and biochemical parameters would be repeated after resolution of acute illness, to assess the cause of anaemia.
The association between severe malnutrition and ID is of clinical significance. As noted, four study participants had severe malnutrition with oedema and 23 had severe malnutrition. The results showed that all of the study participants with severe malnutrition with oedema had IDA and a CHr <29 pg, and of the 23 participants with severe malnutrition, 14 (61%) had results suggestive of ID (i.e. TfS <25% with CHr <29 pg). Iron supplementation in the rehabilitation phase, as advocated by the WHO's 10 steps of managing severe malnutrition, is therefore justified.
Anaemia is a common occurrence in children with HIV infection, not only secondary to the disease, but also associated with ART.[19] The study had 12 participants who were HIV-reactive. This group's results suggested anaemia of chronic disease. In anaemia of chronic disease, as for instance with HIV infection, S-iron, S-transferrin and TfS levels are reduced, with a normal to high S-ferritin level. The seven cases observed with a low CHr level might have been attributed to the presence of both ID and anaemia of chronic illness.[16]
It is our opinion that CHr will be an accurate and cost-effective marker for diagnosing ID in acutely ill infants and children. CHr interpreted together with the full blood count has a high sensitivity. Besides being falsely elevated in iron-deficient children with underlying disease, the assessment of S-ferritin costs approximately three times as much as that of CHr. When an infant/child is assessed to be iron-deficient on the basis of CHr, the S-ferritin level can be determined after recovery from acute illness to confirm the presence of ID.
The prevalence of ID in our sample, based on TfS, was 82% (95% margin of error ±7.53%), with 70.7% of these children having IDA. This represents a significant amount of children who require iron supplementation and regular follow-up to evaluate their iron status in order to prevent neurodevelopmental sequelae such as learning delay, poor socio-emotional performance and sleep disturbances.
Study limitations
Study limitations included the broad age categories of the cohort, and the small size of the sample owing to financial constraints. Further studies are needed with larger samples and narrower age categories to assess the sensitivity and specificity of CHr as a diagnostic test for ID in acutely ill children.
Conclusion
The results of our study support the evidence that CHr is accurate in the diagnosis of ID, and of IDA in combination with a low Hb level, in ill, hospitalised infants and children. After an infant or child has recovered from their acute illness, S-ferritin can be determined to support the diagnosis.
Funding acknowledgement
This study was funded by PathCare laboratories, Bloemfontein, and Roche, SA.
Acknowledgements
We extend our gratitude to all caregivers and children who participated in the study.
References
1. Petit K, Rowley J, Brown N. Iron deficiency. Paediatr Child Health 2011;21(8):339-343. [http://dx.doi.org/10.1016/j.paed.2011.03.006] [ Links ]
2. Chang S, Wang L, Wang Y, et al. Iron-deficiency anaemia in infancy and social emotional development in preschool-aged chinese children. Pediatrics 2011;127:e927-e933. [http://dx.doi.org/10.1542/peds.2010-1659] [ Links ]
3. Gupta SK, Bansal D, Malhi P, Das R. Developmental profile in children with iron deficiency anemia and its changes after therapeutic iron supplementation. Indian J Pediatr 2010;77:375-379. [http://dx.doi.org/10.1007/s12098-010-0046-9] [ Links ]
4. Carter RC, Jacobson JL, Burden MJ, et al. Iron deficiency anemia and cognitive function in infancy. Pediatrics 2010;126(2):427-433. [http://dx.doi.org/10.1542/peds.2009-2097] [ Links ]
5. Peirano PD, Algarin CR, Chamorro RA, et al. Sleep alterations and iron deficiency anemia in infancy. Sleep Med 2010;11:637-642. [http://dx.doi.org/10.1016/j.sleep.2010.03.014] [ Links ]
6. Ziaullah K, Nawaz S, Shah S, Talaat A. Iron deficiency anemia as a cause of breath holding spells. JPMI 2005;19(2):171-174. [ Links ]
7. Beri S, Kahn A, Hussain N, Gosalakkal J. Case report: Severe anemia causing cerebral venous sinus thrombosis in an infant. J Pediatr Neurosci 2012;7:30-32. [http://dx.doi.org/10.4103/1817-1745.97619] [ Links ]
8. Kaul B, Ramanarayanan S, Mahapatra H, Sethi TK, Ahlawat R. Iron deficiency masquerading as idiopathic intracranial hypertension. BMJ Case Rep 2009. [http://dx.doi.org/10.1136/bcr.06.2008.0346] [ Links ]
9. Berry L, Hendricks M. Nutrition-Iron Deficiency Anaemia in Children. Cape Town: Children's Institute. University of Cape Town, 2009. [ Links ]
10. Skikne B, Hershko C. Iron Deficiency. In: Anderson GJ, McLaren G, eds. Iron Physiology and Pathophysiology in Humans. New York: Springer Science, 2012:251-277. [ Links ]
11. Kiudeliene R, Griniute R, Labanauskas L. Prognostic value of reticulocyte hemoglobin content to diagnose iron deficiency in 6-24 month old children. Medicana 2008;44:9:673-677. [ Links ]
12. Orkin SH, Nathan DG, Ginsburg D, et al. Nathan and Oski's Hematology of Infancy and Childhood. 6th ed. Philadelphia: Saunders, 2003:468. [ Links ]
13. Bakr A, Sarette G. Measurement of reticulocyte hemoglobin content to diagnose iron deficiency in Saudi Children. Eur J Pediatr 2006;165:442-445. [http://dx.doi.org/10.1007/s00431-006-0097-0] [ Links ]
14. Ullrich C, Wu A, Armsby C, et al. Screening healthy infants for iron deficiency using reticulocyte hemoglobin content. JAMA 2005;294(8):924-930. [http://dx.doi.org/10.1001/jama.294.8.924] [ Links ]
15. Marshall WJ. Clinical Chemistry. 4th ed. Edinburgh: Mosby, 2000:283-284. [ Links ]
16. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med 2005;352(10):1011-1023. [http://dx.doi.org/10.1056/NEJMra041809] [ Links ]
17. Mackinnon A. A spreadsheet for the calculation of comprehensive statistics for the assessment of diagnostic tests and inter-rater agreement. Computers in Biology and Medicine 2000;30(3):127-134. [ Links ]
18. Wu A, Lesperance L, Bernstein H. Screening for iron deficiency. Pediatr Rev 2002;23(8):171-177. [http://dx.doi.org/10.1542/pir.23-5-171] [ Links ]
19. Eley BS, Sive AA, Shuttleworth M, Hussey GD. A prospective, cross-sectional study of anaemia and peripheral iron status in antiretroviral naive, HIV-1 infected children in Cape Town, South Africa. BMC Infect Dis 2002;2:3. [http://dx.doi.org/10.1186/1471-2334-2-3] [ Links ]
 Correspondence:
Correspondence:
P D R Swart
(riekert.swart@gmail.com)














