Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.7 n.4 Pretoria Apr. 2013
RESEARCH
Respiratory outcomes following 100 mg/kg v. 200 mg/kg of poractant alpha: A retrospective review
E CloeteI, II ; C LoIII; M J BukshIV, V
IMB ChB, FRACP; Auckland City Hospital Newborn Services, Auckland, New Zealand
IIMB ChB, FRACP; School of Medicine, University of Auckland, New Zealand
IIIMN (Neonatal); Auckland City Hospital Newborn Services, Auckland, New Zealand
IVMBBS, FRACP; Auckland City Hospital Newborn Services, Auckland, New Zealand
VMBBS, FRACP; School of Medicine, University of Auckland, New Zealand
ABSTRACT
BACKGROUND: The treatment guideline for the management of respiratory distress syndrome in the newborn unit at Auckland City Hospital (ACH), Auckland, New Zealand, was amended in July 2010. In keeping with current evidence, the initial dose of poractant alpha was increased from 100 mg/kg to 200 mg/kg. The outcomes of newborns requiring treatment with surfactant before and after this change were reviewed.
METHODS: Electronic clinical records were reviewed of infants admitted to ACH who received surfactant during the period December 2008 - December 2011. There were two groups: group A were infants who received 100 mg/kg of poractant alpha as an initial dose (December 2008 - June 2010), and group B were infants who received 200 mg/kg as an initial dose (July 2010 - December 2011). Infants with congenital anomalies and those treated with surfactant before transfer to ACH were excluded.
RESULTS: A total of 256 infants were included in the analysis, 118 in group A and 138 in group B. Infants in group B had a higher median gestational age (28 v. 27 weeks; p=0.52) and birth weight (1 065 g v. 930 g; p=0.08) compared with infants in group A. Significantly more infants in group A received more than one dose of surfactant (33.9% v.15.9%; odds ratio 2.7; p=0.0008). Infants in group B showed a signiicant reduction in oxygen requirement ater the administration of surfactant (p=0.0003).
CONCLUSION: The administration of 200 mg/kg poractant alpha led to a significant improvement in oxygenation and a reduction in the need for further doses of surfactant.
Surfactant replacement therapy has revolutionised neonatal respiratory care over the past two decades. Whether given prophylactically or as rescue therapy to newborns with or at risk of developing respiratory distress syndrome, it reduces the incidence of air leaks and improves survival.[1-5] It has also been shown to improve ventilation and oxygenation in the first 48 - 72 hours after birth.[6]
According to the European Consensus Guidelines on the Management of Neonatal Respiratory Distress Syndrome in Preterm Infants,[1] at least 100 mg/kg of phospholipid is required for the management of neonatal respiratory distress syndrome. Recent pharmacokinetic and clinical data suggest that a dose of 200 mg/kg of phospholipid has a longer half-life and is associated with a better acute response and a reduced need for subsequent doses of surfactant therapy than lower doses.[1,7]
Poractant alpha (Curosurf), a natural porcine-derived surfactant, is the preparation used by the newborn services at Auckland City Hospital (ACH), Auckland, New Zealand. In our unit the following infants qualify for a dose of surfactant: (i) infants ≤30 weeks' gestational age (GA) who require ventilation; and (ii) infants >30 weeks' GA with clinical, radiological and laboratory findings suggestive of moderate to severe surfactant deficiency. The guideline for administering poractant alpha for the management of respiratory distress syndrome in neonates at ACH was amended in July 2010.[8] Before this, infants were given an initial dose of 100 mg/kg. The dose remained 100 mg/kg when a second or third dose was administered. From July 2010, infants have been given 200 mg/kg as an initial dose and 100 mg/kg subsequently if further doses are required. The current guideline is in accordance with the recommendations from the manufacturer of poractant alpha[9] as well as the consensus guideline.[11] The unit's criteria for administering a second dose of surfactant (the need for positive-pressure ventilation and/or an oxygen requirement >30%[8]) have remained unchanged.
In this retrospective study, we compared the outcomes of a cohort of infants treated with an initial dose of 200 mg/kg poractant alpha after our amended guideline came into effect with a historical group of similar infants treated with 100 mg/kg.
Methods
This was a retrospective review of respiratory outcomes of neonates receiving treatment with surfactant over a 3-year period. Data were collected from ACH electronic medical records and the newborn services database. Infants admitted to the newborn unit at ACH who received at least one dose of poractant alpha during the period December 2008 - December 2011 were identified from the database and divided into two groups: group A were infants who received 100 mg/kg of poractant alpha as an initial dose (December 2008 - June 2010), and group B were infants who received 200 mg/kg as an initial dose (July 2010 - December 2011).
Infant records were reviewed by a neonatal fellow and a neonatal nurse specialist. The primary outcomes were: (i) duration of oxygen supplementation; including the need for and duration of home oxygen; (ii) the change in fraction of inspired oxygen (FiO2) following the first dose of surfactant (FiO2 immediately before and 1 hour after the administration of surfactant was recorded; oxygen delivery aimed to keep saturation levels within the target range[10]); (iii) the need for subsequent doses of surfactant and the time to administration; (iv) duration of mechanical ventilation; (v) rates of pneumothoraces; and (vi) death due to all causes. Secondary outcomes reviewed were: (i) major cerebral abnormality on head ultrasound (periventricular leukomalacia, hydrocephalus, grade 3 or 4 intraventricular haemorrhage); (ii) pulmonary haemorrhage (presence of fresh blood up endotracheal tube); (iii) necrotising enterocolitis (Bell stages IIA - IIIB); (iv) retinopathy of prematurity (stage ≥ 3); (v) acquired pneumonia or sepsis (culture proven); (vi) patent ductus arteriosus (treated medically or surgically); and (vii) duration of hospital stay.
Data were analysed using JMP V10.0 (SAS Inc.) statistical software. Results from the two groups were compared using Pearson’s chi-square test for categorical measures and Wilcoxon’s rank sum test or analysis of variance, as appropriate, for continuous measures. Odds ratio (OR) p-values were likelihood ratio based. Adjustments for confounders were undertaken using multivariable logistic regression.
Ethics approval was obtained from the Northern X Regional Ethics Committee, New Zealand.
Results
A total of 3044 infants were admitted to the ACH newborn unit over the 36-month period 1 December 2008 - 31 December 2011. Of these infants, 570 (18.7%) had a GA of <32 weeks. A database search identified 277 infants (9.1% of total admissions) who received treatment with surfactant during this period, 128 in group A and 149 in group B. After excluding infants with congenital anomalies and those who were treated with surfactant before transfer to ACH, a total of 256 infants were included in the analysis, 118 in group A and 138 in group B. Patient characteristics are described in Table 1. Infants in group B had a higher median GA based on early antenatal scan findings (28 v. 27 weeks; p=0.52) and birth weight (BW) (1065 g v. 930 g; p=0.08) than infants in group A; furthermore, 85.6% of infants in group A had a very low BW (<1500 g) compared with 75.4% of infants in group B (Fig. 1). Gender distribution, the number of multiple births, births by caesarean section and the number of infants who received a complete course of antenatal steroids were similar in the two groups (Table 1).
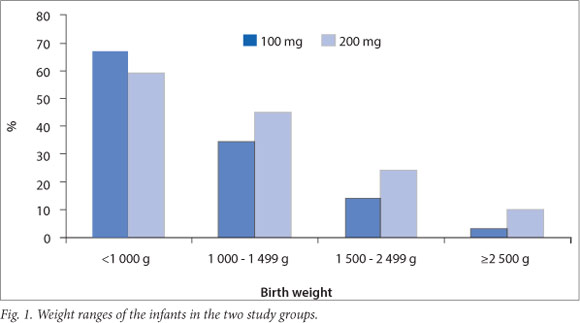
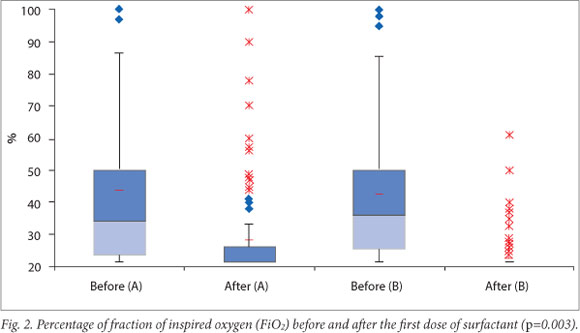
Results for four primary outcomes are shown in Table 2. There were no statistically significant differences between the two groups for any of these outcomes. There was, however, a trend towards shorter duration of mechanical ventilation as well as lower rates of pneumothoraces and mortality in group B. Infants in group B showed a significantly greater reduction in oxygen requirement after the administration of surfactant (p=0.0003).
Fig. 2 shows oxygen requirements of the two groups of infants before and after receiving the initial treatment dose of surfactant.
Sixty-three (24.6%) of the 256 infants received more than one dose of surfactant. Significantly more infants in group A than in group B received more than one dose of surfactant (33.9% v.15.9%; OR 2.7; p=0.0008). Adjusting for BW and GA made no material difference (OR 2.9; p=0.0007). Four patients (3.4%) in group A required a third dose of surfactant, but none of the infants in group B was treated with more than two doses.
Fifty-seven per cent of infants in group A and 29% in group B received the first dose of surfactant within 15 minutes of birth. Eighty-six infants, 44 in group A (37.3%) and 42 in group B (30.4%), received the first dose beyond 2 hours of age. The median time to the administration of the second dose was 10 hours in group A and 12 hours in group B.
We performed a subgroup analysis comparing the outcomes for very-low-birthweight (VLBW) infants in the two groups (Table 3). There were 101 infants in group A and 104 in group B with a BW of <1 500 g. In this cohort the percentage of infants who required respiratory support beyond 28 days of age was higher and the median duration of ventilation was longer compared with the cohort as a whole. Rates of pneumothoraces were significantly (p=0.05) lower in infants who received 200 mg/kg of surfactant than in those who received 100 mg/kg. There were no other significant differences between the two groups.
We also compared the outcomes for the infants in the two groups who were treated with a single dose of surfactant (Table 4). There were no statistically significant differences between these groups.
The mortality rate was significantly higher among infants who required more than one dose of surfactant, regardless of the size of the initial dose (27.5% of infants in group A and 22.7% of infants in group B who required more than one dose of surfactant died). Results of the secondary outcomes are shown in Table 5. Significantly fewer infants in group B received treatment for patent ductus arteriosus (p=0.008). There were no other significant differences between the groups. Theese complications are mostly associated with VLBW infants, so it made no material difference when infants with a BW >1500 g were excluded from the analysis. The median (range) duration of hospital stay was 87 days (24 - 141 days) for VLBW infants in group A and 86 days (20 - 196 days) for group B (p=0.33).
Discussion
Safe and efective surfactant replacement therapy has been in use since the early 1990s.[2,11,12] More recent studies have focused on establishing the optimal dose. Randomised controlled trials have shown that multiple doses of surfactant reduce rates of air leak syndromes and mortality among infants at risk of or with established respiratory distress syndrome.[13-16] A wide range of dosing schedules as well as different types of surfactant have been used in these studies.
The results of a survey conducted across 173 neonatal units in Europe to determine current clinical practice relating to surfactant administration were published in 2011.[17] Poractant alpha was the most commonly used surfactant preparation and was used by 148 units (86%). The most frequently used initial dose was 100 mg/kg (58%), followed by 200 mg/kg (39%). Doses of 200 mg/kg were only administered when using poractant alpha. Forty-three per cent of infants in this survey received a second dose of surfactant. It is not stated what percentage of these infants received 200 mg/kg as a first dose. We have shown that infants who received 200 mg/kg of poractant alpha are less likely to require additional doses than infants who receive a smaller initial dose. Sixty-three (24.6%) of the 256 infants in our study received more than one dose of surfactant. Significantly fewer infants required a second dose after initial treatment with 200 mg/kg (15.9% v. 33.9% of infants who received 100 mg/kg; p=0.0008). Cogo et al.[7] compared outcomes in 61 preterm infants with respiratory distress syndrome treated with poractant alpha. In their study, 70% of infants required a second dose of surfactant after an initial dose of 100 mg/kg compared with only 28.6% of infants who received 200 mg/kg. Similar to our findings, most infants who required a third dose had received 100 mg/kg as an initial dose (22.5%). Only 1 infant in their 200 mg group received three doses.
We found a mortality rate of 14.4% in group A compared with 8.7% in group B. Ramanathan et al.[18,19] reported a 3% mortality rate in infants treated with 200 mg/kg of poractant alpha v. 11% for infants treated with 100 mg/kg of either beractant or poractant alpha. We found a significantly higher mortality rate in infants who received more than one dose of surfactant, regardless of whether they received 100 mg/kg or 200 mg/kg as the initial dose. The mortality rate was, however, consistently higher among the 100 mg group. It is well recognised that BW and GA at birth have the greatest impact on all outcomes, including respiratory distress syndrome and mortality. However, exclusion of infants with a BW of >1 500 g did not alter this finding. The mortality rate in the VLBW cohort was 15.8% in group A and 10.6% in group B.
Many of the 63 infants in our study who required more than one dose of surfactant continued to have a complex course throughout their hospital stay. This group was ventilated for a longer period, spending a median of 7.66 days on the ventilator compared with 1.04 days for the 194 infants who received only a single dose of surfactant. Rates of sepsis, necrotising enterocolitis and major cerebral abnormalities were also higher among these infants. The most likely explanation for this observation is that the infants who received second or third doses of surfactant were a select group who were sicker and therefore more likely to do poorly overall.
We found that fewer infants received surfactant within 15 minutes after birth in group B than in group A (29% v. 57%). Although no other changes were made to our unit's surfactant administration guidelines during the study period, there has been a movement towards managing infants with continuous positive airway pressure and, if required, early rescue with surfactant rather than administration of prophylactic surfactant to infants at risk of developing respiratory distress syndrome. This may account for some of the infants receiving surfactant later.
Cogo et al.[7] investigated the kinetics of surfactant disaturated phophatidylcholine and found a significantly longer half-life in the 200 mg/kg group (32±19 hours) compared with the 100 mg/kg group (15±15 hours). We found that the interval between the first and second doses of surfactant tends to be longer after the administration of 200 mg/kg. Infants in the 200 mg group who required a second dose received it at a median time of12 hours compared with 10 hours for the 100 mg group.
We have shown a trend towards shorter ventilation times, shorter hospital stays and lower mortality among infants who received 200 mg/kg of surfactant as an initial dose compared with 100 mg/kg. Although none of these findings reached statistical significance, they have clinical significance. In some clinical settings it may mean having a ventilator or cot space available for another sick infant. We have also shown a clear reduction in rates of patent ductus arteriosus requiring treatment and in the incidence of pneumothoraces among VLBW infants treated with 200 mg/kg of surfactant.
This study has a number of limitations. Data were retrospectively collected from electronic records, and the accuracy thereof therefore depends on the quality of record keeping. We were, however, able to obtain relevant data on all outcomes of interest in this study. The study sample size was small, and the results should therefore be interpreted with caution.
Conclusion
In this retrospective study, we found that there was a significant improvement in oxygenation and a marked reduction in the need for further doses of surfactant after the administration of 200 mg/ kg of poractant alpha compared with 100 mg/kg. These findings are in keeping with current literature and support the current guideline for the management of newborn infants with respiratory distress syndrome at ACH.
Acknowledgements. We thank the Children's Research Centre at Starship Children's Hospital for statistical advice. EC received a fellowship sponsored by the Starship Foundation.
References
1. Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants - 2013 update. Neonatology 2013;103(4):353-368. [http://dx.doi.org/10.1159/000349928] [ Links ]
2. Engle WA. Surfactant-replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics 2008;121(2):419-432. [http://dx.doi.org/10.1542/peds.2008-0910] [ Links ]
3. Soll RF. Prophylactic synthetic surfactant for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2000(2):CD001079. [http://dx.doi.org/10.1002/14651858.CD001079] [ Links ]
4. Soil RF, Blanco F. Natural surfactant extract versus synthetic surfactant for neonatal respiratory distress syndrome. Cochrane Database Syst Rev 2001(2):CD000144. [http://dx.doi.org/10.1002/14651858.CD000144] [ Links ]
5. Sweet DG, Halliday HL. The use of surfactants in 2009. Arch Dis Child Educ Pract Ed 2009;94(3):78-83. [http://dx.doi.org/10.1136/adc.2008.153023] [ Links ]
6. Soll RF. Prophylactic natural surfactant extract for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev 2000(2):CD000511. [http://dx.doi.org/10.1002/14651858.CD000511] [ Links ]
7. Cogo PE, Facco M, Simonato M, et al. Dosing of porcine surfactant: Effect on kinetics and gas exchange in respiratory distress syndrome. Pediatrics 2009;124(5):e950-957. [http://dx.doi.org/10.1542/peds.2009-0126] [ Links ]
8. Auckland City Hospital Newborn Services, Auckland, New Zealand. Poractant alpha (Curosurf) (Drug protocol). 2010 (updated July 2010). http://www.adhb.govt.nz/newborn/DrugProtocols/PoractantPharmacology.htm (accessed 20 June 2013). [ Links ]
9. Chiesi Farmaceutici, Parma, Italy. Curosurf (poractant alpha) intratracheal suspension. 2012. http://crtx.com/docs/curosurf_PI.pdf (accessed 27 August 2012). [ Links ]
10. Auckland City Hospital Newborn Services, Auckland, New Zealand. Oxygen saturations and targeting. 2013 (updated February 2013). http://www.adhb.govt.nz/newborn/Guidelines/Respiratory/Oxygen/OxygenSaturation Targets.htm (accessed 3 July 2013). [ Links ]
11. Cotton RB, Olsson T, Law AB, et al. The physiologic effects of surfactant treatment on gas exchange in newborn premature infants with hyaline membrane disease. Pediatr Res 1993;34(4):495-500. [http://dx.doi.org/10.1203/00006450-199310000-00022] [ Links ]
12. Halliday HL. Surfactants: Past, present and future. J Perinatol 2008;28(Suppl 1):S47-56. [http://dx.doi.org/10.1038/jp.2008.50] [ Links ]
13. Speer CP, Robertson B, Curstedt T, et al. Randomized European multicenter trial of surfactant replacement therapy for severe neonatal respiratory distress syndrome: Single versus multiple doses of Curosurf. Pediatrics 1992;89(1):13-20. [http:dx.doi.org/10.1203/00006450-199009000-00052] [ Links ]
14. Soll R. Multiple versus single dose natural surfactant extract for severe neonatal respiratory distress syndrome. Cochrane Database Syst Rev 1999(2):CD000141. [http://dx.doi.org/10.1002/14651858.CD000141] [ Links ]
15. Dunn MS, Shennan AT, Possmayer F. Single- versus multiple-dose surfactant replacement therapy in neonates of 30 to 36 weeks' gestation with respiratory distress syndrome. Pediatrics 1990;86(4):564-571. [ Links ]
16. Corbet A, Gerdes J, Long W, et al. Double-blind, randomized trial of one versus three prophylactic doses of synthetic surfactant in 826 neonates weighing 700 to 1100 grams: Effects on mortality rate. American Exosurf Neonatal Study Groups I and IIa. J Pediatr 1995;126(6):969-978. [http://dx.doi.org/10.1016/S0022-3476(95)70226-1] [ Links ]
17. Van Kaam AH, De Jaegere AP, Borensztajn D, Rimensberger PC. Surfactant replacement therapy in preterm infants: A European survey. Neonatology 2011;100(1):71-77. [http://dx.doi.org/10.1159/000322004] [ Links ]
18. Ramanathan R, Rasmussen MR, Gerstmann DR, Finer N, Sekar K. A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants. Am J Perinatol 2004;21(3):109-119. [http://doi.org/10.1055/s-2004-823779] [ Links ]
19. Ramanathan R, Bhatia JJ, Sekar K, Ernst FR. Mortality in preterm infants with respiratory distress syndrome treated with poractant alfa, calfactant or beractant: A retrospective study. J Perinatol 2013;33(2):119-125. [http://doi.org/10.1038/jp.2011.125] [ Links ]
 Correspondence: E Cloete (elzac@adhb.govt.nz)
Correspondence: E Cloete (elzac@adhb.govt.nz)














