Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.7 n.3 Pretoria Jan. 2013
RESEARCH
Characteristics of children with pervasive developmental disorders attending a developmental clinic in the Western Cape Province, South Africa
P E SpringerI; R van ToornII ; B LaughtonIII; M KiddIV
IDCH (SA), FCPaed (SA), Department of Paediatrics and Child Health, Tygerberg Hospital and Faculty of Health Sciences, Stellenbosch University, Parow, Cape Town, South Africa
IIFCPaed (SA) Department of Paediatrics and Child Health, Tygerberg Hospital and Faculty of Health Sciences, Stellenbosch University, Parow, Cape Town, South Africa
IIIDCH (SA), FCPaed (SA), MSc Med (Child Health Neurodevelopment) Department of Paediatrics and Child Health, Tygerberg Hospital and Faculty of Health Sciences, Stellenbosch University, Parow, Cape Town, South Africa
IVBSc Hons, MSc, PhD (Statistics), Centre for Statistical Consultation, Stellenbosch University, Stellenbosch, Western Cape, South Africa
ABSTRACT
BACKGROUND: Little has been published on autism in Africa, and it is not known whether South African children present with the same characteristics and challenges as described internationally.
OBJECTIVES: To describe the demographics, history, clinical features, co-morbidity and yield of aetiological investigations in children diagnosed with a pervasive developmental disorder (PDD).
METHODS: This was a retrospective review of medical records of children fulfilling Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR) criteria for a PDD who attended a tertiary developmental clinic at Tygerberg Hospital, Western Cape, South Africa, over a 2-year period (2008 - 2010).
RESULTS: Fifty-eight children were included. The median age at diagnosis was 42 months (range 15 - 106 months), and 45 (77.6%) were boys. Forty per cent had complex autism (dysmorphism with or without microcephaly), and 12.1% were macrocephalic. Most children (72.4%) were non-verbal (using fewer than 10 non-echoed words), and 89.0% had behavioural problems as reported by caregivers. The diagnostic yield of investigations was low.
CONCLUSION: The profile of children with PDD attending a tertiary hospital developmental clinic in the Western Cape revealed that a high proportion had severe language impairment, behavioural problems and complex autism.
Despite the fact that autism affects children worldwide, most of what is known about infant mental health originates from developed countries. This imbalance in knowledge is emphasised by a study that surveyed 764 journal articles on infant behaviour and development and found that 78% were from North America, 16% from Europe and only 4% from the rest of the world.[1] There are few published studies on autism in Africa.[2]
The prevalence of the pervasive developmental disorders (PDDs) or autism spectrum disorder (ASD) in South Africa is unknown. It is important to note that in some countries ASD refers only to autistic disorder, Asperger syndrome, and pervasive developmental disorder not otherwise specified (PDD-NOS).[3] In others, including South Africa, the terms ASD and PDD are still used interchangeably,[4] and for the purpose of this study we too have elected to consider the term ASD as synonymous with PDD.
In 2007 the point prevalence of ASD diagnosis in US children was estimated to be 110/10 000.[5] This implies there could potentially be over 270 000 people with ASD in South Africa, with a predicted 5 000 new cases per year. Epidemiological surveys in the USA since the early 1980s have revealed that increasing numbers of children are being diagnosed with ASD.[5] A similar trend was described in a South African study that documented an 8.2% increase in the number of children presenting with features of ASD to a developmental clinic in Johannesburg over the period 1996 - 2005.[6] It is not clear whether the increasing rate of the disorder is related to broadening of the diagnostic criteria or heightened awareness of the condition among parents and professionals. Studies have yet to be done to determine the prevalence and characteristics of ASD in Africa.
Objectives
We aimed to add to the knowledge of autism in South Africa by describing the demographics, history, clinical features, co-morbidity and diagnostic yield of investigations in a group of children diagnosed with a PDD at a tertiary developmental paediatric clinic.
Methods
The Tygerberg Children's Hospital (TCH) developmental clinic provides a tertiary consultative service to mainly preschool children living in the Western Cape, South Africa. Children are referred from primary and secondary public health facilities, but a small proportion of referrals originate from private healthcare practitioners, speech or occupational therapists and teachers. The majority of patients accessing public health services are of low socio-economic status.
This descriptive study involved a retrospective review of medical records over 2 years (1 April 2008 - 31 March 2010). All children attending the TCH developmental assessment clinic who fulfilled Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision (DSM-IV-TR) criteria for PDD were included.[7] Children presenting with severe cognitive disability, limited communication or social interaction deficits without meeting the full criteria for PDD were excluded, as were those with inconclusive or incomplete information.
All children identified from the medical record review were seen by a single developmental paediatrician (PES) who interviewed primary caregivers about the child's medical and behavioural history, undertook a clinical examination and developmental assessments, and observed the child's play and behaviour. The paediatrician assessed each child even if the diagnosis of PDD had been made by another clinician at an earlier visit.
All the children underwent hearing and speech assessment. Where possible, additional input was obtained from the occupational therapist, preschool teacher and other professionals working with the child. The Childhood Autism Rating Scale (CARS) was utilised in cases of diagnostic uncertainty.[8] Diagnostic instruments such as the Autism Diagnostic Observation Schedule (ADOS) were not available at the time of the study owing to resource limitations.
Children were classified as 'verbal' (using 10 or more meaningful, non-echoed words) or 'non-verbal' (using fewer than 10 words) according to information obtained from their caregivers.
We used the Molteno Adapted Scale,[9] a developmental screening tool used in the Western and Eastern Cape that allows estimation of age equivalents in each of four developmental domains, i.e. gross motor, fine motor, language and personal/social. The age equivalents were used to estimate both subquotients and a general developmental quotient (DQ). Children who had delays in two or more developmental domains were categorised as having global developmental delay. This was further classified as mild if the DQ was in the range 51 - 70, moderate if the DQ was 31 - 50, and severe if the DQ was <30. Psychometric assessments on children older than 6 years were included if available.
Microcephaly was defined as a measured head circumference below the 2nd percentile and macrocephaly as a circumference above the 98th percentile.[10]
Children with evidence of abnormal early embryonic development, including dysmorphism and/or microcephaly, were categorised as having 'complex autism' and the remainder as having 'essential autism, as previously described by Miles et al.[11]
Information captured on a data sheet included demographics, history including commonly described risk factors,[3,12-18] clinical characteristics, co-morbidity and special investigations.
Statistical analysis
Summary statistics were reported as frequencies and percentages. A Χ2 square test was conducted to establish different verbal ability among ethnic groups.
Ethical approval
The study was approved by the Health Research Ethics Committee of Stellenbosch University (ethics approval number N10/10/336).
Results
From the 1 010 clinic files reviewed from the study period, 75 children were described as having autistic features listed in the DSM-IV-TR, but only 58 met the full DSM-IV-TR criteria for PDD. Of the children excluded, 9 had severe cognitive disability and social interaction deficits but did not meet all the criteria for PDD, and in 8 cases there was dispute or insufficient documentation to make the diagnosis. On further analysis, 55 of the 58 children could be sub-classified as having autistic disorder (Table 1), and there was 1 case each of Asperger syndrome, PDD-NOS and Rett syndrome. Of the 1 010 files reviewed, 58 (5.7%) therefore met the criteria for a PDD (ASD).

Table 2 illustrates the demographics of the study participants and information received from the caregivers. The median age at PDD (ASD) diagnosis was 42 months, and the majority were boys (77.6%). Just over half were of mixed South African ancestry. Of those with documented parental ages, 17.2% had mothers who were 35 years or older at the time of the child's birth. An education level of senior school certificate (matric) or higher was documented in 46.6% of mothers and 44.8% of fathers.
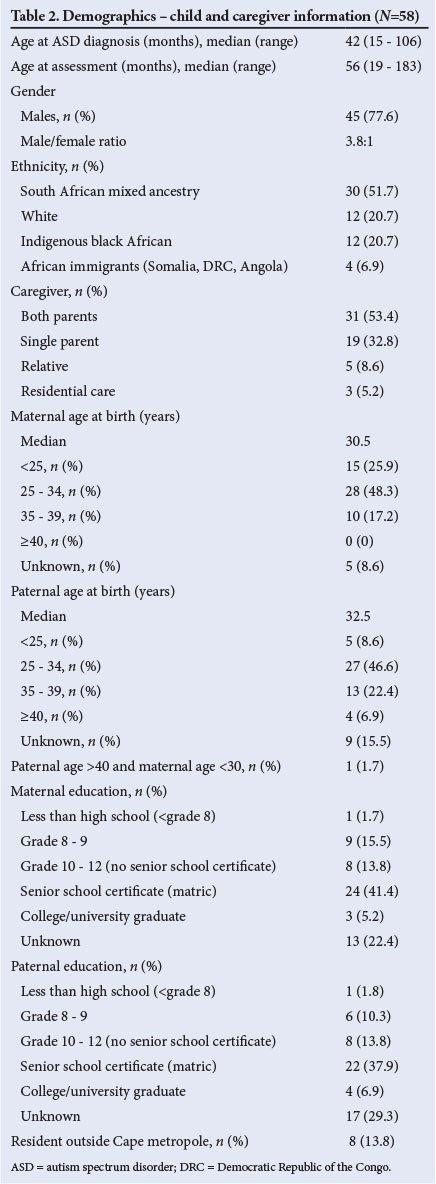
Table 3 documents the participants' histories, commonly described risk factors, additional health problems and problematic behaviours; there was a history of emotional or social stress during pregnancy and/or birth in 17.2%, and a history of prenatal toxin exposure in 8.6% (1 mother admitted to using alcohol and methamphetamines).
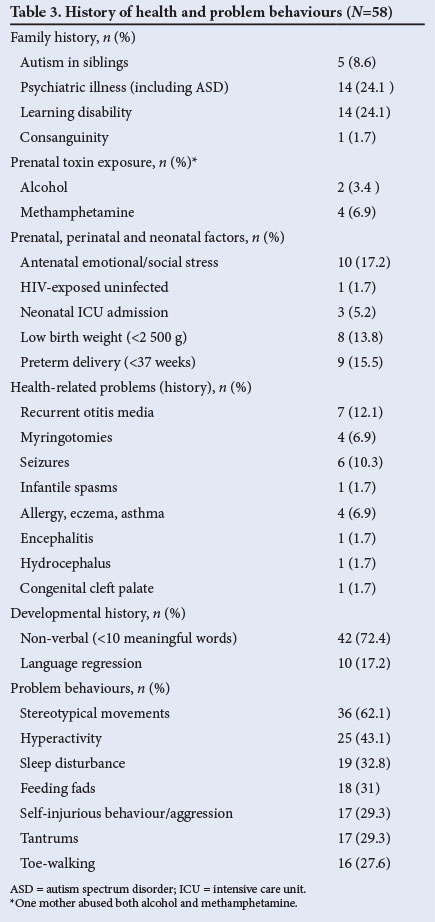
Eight per cent of children had a sibling with a diagnosis of ASD, and there was a family history of psychiatric illness in 24.1% and of learning disabilities in 24.1%. Eighty-nine per cent of the children were reported to have at least one of the behavioural problems listed in Table 3. The majority of the children were non-verbal (72.4%), a significantly higher proportion of black African children (93.7%) being non-verbal at presentation compared with children of mixed ancestry (76.7%) and white children (41.7%) (p=0.005).
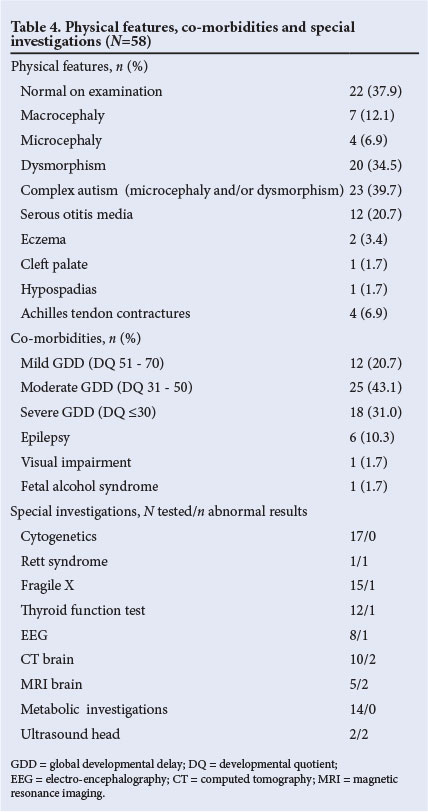
Table 4 shows the subjects' physical features, co-morbidities and the diagnostic yield of special investigations. Thirty-eight per cent had normal findings on examination, 34.5% had dysmorphic features, 6.9% were microcephalic and 12.1% were macrocephalic. Complex autism (microcephaly and/or dysmorphism) generally associated with abnormal morphogenesis was therefore present in about 40%. The most common co-morbidity was global developmental delay, with 43.1% having moderate and 31.0% severe delay. Only 2 children, both older than 6 years, had undergone formal psychometric assessment (the child with Asperger syndrome and another with mild intellectual disability). The diagnostic yield of genetic and metabolic investigations was low.
Discussion
The male predominance (77.6%) was similar to that reported in a South African study in KwaZulu-Natal.[19] The ethnic groups represented the demographic profile of the hospital drainage area. The black African group had the highest proportion of non-verbal children. Health professionals readily refer non-verbal children to rule out hearing impairment, but it is possible that verbal children presenting with milder degrees of autism were overlooked or not referred. There is a lack of evidence in the literature to support a genetic causation of observed ethnic differences in autism prevalence. Zaroff and Uhm[20] concluded that racial variations were probably due to methodological and socio-economic variables rather than being true ethnic differences. It is possible that variations in help-seeking behaviour or even accessibility of the TCH developmental clinic may have contributed to our findings.
Median age at diagnosis of ASD in our cohort was similar to that of children attending a tertiary developmental clinic in Singapore. This study linked younger age at diagnosis with severity of autism.[21] Moderately severe and severe autism was deemed to be present in 86% of children with a mental development index on the Bayley Scales of Infant Development of <50 or whose compliance with cognitive assessment was poor as a result of autistic features. In our study 74.1% of children had a DQ of <50, which would place them in this category.
The level of parental education in the study group was relatively high, with 60.3% of mothers having attained a minimum of grade 10 compared with 44.8% in another cohort of children attending the hospital.[22] Education may have resulted in an increased awareness of developmental delay, different help-seeking strategies, and ultimately improved ability to gain early access to the clinic.
Studies on autism and parental age have yielded conflicting results on whether mothers, fathers or both contribute to increased risk. Recent studies found that a woman's risk of giving birth to a child who develops autism increases throughout her reproductive years. However, while paternal age confers an increased risk of autism when mothers are 30 years old or younger, it has little effect when mothers are past age 30.[12] There was only 1 participant with a father older than 40 years and a mother younger than 30 years; however, paternal age was undocumented in 15.5% of the fathers in our study. A history of consanguinity was obtained in only 1 family, which contrasts with the high rate of consanguinity (39.3%) described in a Tunisian ASD study.[23]
The prevalence of low birth weight in the study participants was not significantly different from that in the Western Cape population (17%).[24] A number of studies have investigated the association between pre-, peri- and neonatal factors and autism.[13] They report a consistent association between unfavourable events in pregnancy, delivery and the neonatal period and occurrence of PDD. However, Bailey et al. found that 'obstetric hazards appear to be consequences of genetically influenced abnormal development, rather than independent aetiological factors'.[14] Although autism has been associated with preterm birth, it is thought to result from the prenatal and neonatal complications thereof.[15]
Viral infection during early brain development has been proposed as a cause of autism. The best association to date has been made between congenital rubella and autism, but there were no proven cases in our cohort.[16] One study in the literature reported a high prevalence (1 in 95) of autistic behaviour in a sample of 286 children with congenital HIV infection.[17] Surprisingly, considering the high prevalence of HIV infection in the Western Cape, none of the study children was HIV-infected and only 1 child was HIV-exposed but uninfected.
In utero exposures to toxins such as alcohol have recognised association with autism.[18] Alcohol and methamphetamine abuse is problematic in the Western Cape, and 5 mothers in the study (8.6%) admitted to abuse of either alcohol and/or methamphetamine during pregnancy. However, in utero toxin exposure is often under-reported.[25]
Up to 40% of children with ASD experience developmental regression, most often involving loss of speech and/or social responsiveness.[26] The prevalence of developmental regression in our study was 17.2% (n=10), but this may have been an underestimation, as only language regression was documented.
Caregivers reported at least one problematic behaviour in 89.0% of study participants. Stereotypical movements were the most frequent complaint (62.1%), with findings similar to those of Mubaiwa et al.,[19] followed by hyperactivity (43.1%) and sleep disturbance (32.8%). The latter figure was lower than the prevalence of sleep disturbance quoted in the literature (40 - 86%).[27]
Studies on macrocephaly in children with ASD have yielded conflicting results. While earlier studies report a prevalence of macrocephaly as high as 40%,[28] recent studies reported no significant differences in a community-based sample.[29] Our study found a higher rate of macrocephaly (12.1%) compared with the general population (<3%). Microcephaly is present in 5 - 15% of children with autism, and is highly predictive of poor outcome.[28] About 7% of our study children were microcephalic, and one of these children was diagnosed with Rett syndrome. All except 1 were non-verbal, suggesting severe cognitive delay.
Complex autism refers to the presence of dysmorphic features and/ or microcephaly that indicate some alteration of early morphogenesis.
It is found in approximately 20 - 30% of autistic children and is associated with a poor prognosis.[11] Forty per cent of the children in the study group fulfilled criteria for complex autism. This is higher than figures quoted in the literature, but may reflect the referral pattern of the clinic, which included younger children with more severe language delay.
Cognitive ability in children with ASD is difficult to evaluate, especially before intervention, so initial assessment may have been unreliable and not reflected intellectual potential. Formal psychometric testing or use of standardised developmental assessment tools was not possible in the busy clinical setting. However, all but 3 of the children had varying degrees of global developmental delay or intellectual disability and required referral for special school placement. Together with the fact that 72.4% were non-verbal, this suggests a higher proportion of children with intellectual disability than the 50% quoted in the literature, but again probably reflects referral bias.[30] The prevalence of epilepsy (10.3%) corresponds with the literature (5 - 40%).[31] Clinically recognisable syndromes in the study included Rett syndrome, fetal alcohol syndrome and fragile X disorder. In South Africa chromosomal analysis, testing for fragile X and Angelman syndrome and MECP2 investigations are available at most tertiary centres. Cytogenetic and fragile X testing were performed in 29.3%, which is lower than international practice recommendations.[32] The diagnostic yield of all investigations was even lower than reported in the literature.[33]
Limitations of the study include: (i) use of a clinic-based population; (ii) lack of a standardised and validated autism diagnostic tool to confirm ASD/PDD, which may have resulted in exclusion of children with milder forms of autism; (iii) the small number of participants in each ethnic group, making comparisons difficult; (iv) the degree and nature of behavioural problems such as hyperactivity, sleeping and eating disturbance were not always documented; and (v) long-term outcomes to assess the stability of ASD diagnosis are not yet available.
Conclusions
Children attending a tertiary hospital developmental clinic who fulfilled DSM-IV-TR criteria for PDD were a clinically heterogeneous group displaying severe language impairment, problematic behaviour, co-morbidity and complex autism. Most children had problems requiring ongoing medical, social and educational support services.
With regard to special investigations, it may be helpful to focus on children with complex autism (dysmorphism and/or microcephaly).
Increasing public awareness regarding early warning signs of autism will improve early identification of children with ASD. It is anticipated that the recently published DSM-V diagnostic criteria for ASD will increase objectivity and reliability in making the diagnosis.[34]
References
1. Tomlinson M, Swartz L. Imbalances in the knowledge about infancy: The divide between rich and poor countries. Infant Mental Health Journal 2003;24(6):547-556. [http://dx.doi.org/10.1002/imhj.10078] [ Links ]
2. Bakare MO, Munir KM. Autism spectrum disorders (ASD) in Africa: A perspective. Afr J Psychiatry 2011;14(3):208-210. [http://dx.doi.org/10.4314/ajpsy.v14i3.3] [ Links ]
3. Larsson HJ, Eaton WW, Madsen KM, et al. Risk factors for autism: Perinatal factors, parental psychiatric history, and socioeconomic status. Am J Epidemiol 2005;161(10):916-925. [http://dx.doi.org/10.1093/aje/kwi/123] [ Links ]
4. Mubaiwa L. Autism: Understanding basic concepts. South African Journal of Child Health 2008;2(1):6-7. [ Links ]
5. Kogan MD, Blumberg SJ, Schieve LA, et al. Prevalence of parent-reported diagnosis of autism spectrum disorder among children in the US, 2007. Pediatrics 2009;124(5):1395-1403. [http://dx.doi.org/10.1542/peds.2009-1522] [ Links ]
6. Jacklin L. The changing profile of autism in a clinic for children with developmental delay: A ten year survey. Presented at Autism Safari 2006: 2nd World Autism Congress & Exhibition, 30 October - 2 November 2006, Cape Town, South Africa. [ Links ]
7. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed., text revision. Washington, DC: APA, 2000. [ Links ]
8. Schopler E, Reichler R, Renner B. Childhood Autism Rating Scale CARS). 1st ed. Los Angeles: Western Psychological Services, 1988. [ Links ]
9. Laughton B, Dhar P, Springer P, et al. Correlation between the Molteno adapted scale and the Griffiths mental development scales at 11.5 and 21 months. Presented at the Annual Academic Day, Department of Paediatrics, Faculty of Medicine and Health Sciences, Stellenbosch University, Western Cape Province, South Africa, 17 August 2011. [ Links ]
10. Nelhaus G. Head circumference from birth to eighteen years: Practical composite international and interracial graphs. Pediatrics 1968;41(1):106-114. [ Links ]
11. Miles JH, Takahashi TN, Bagby S, et al. Essential versus complex autism: Definition of fundamental prognostic subtypes. Am J Med Genet A 2005;135(2):171-180. [http://dx.doi.org/10.1002/ajmg.a.30590] [ Links ]
12. Shelton JF, Tancredi DJ, Hertz-Picciotto I. Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Res 2010;3(1):30-39. [http://dx.doi.org/10.1002/aur.116] [ Links ]
13. Juul-Dam N, Townsend J, Courchesne E. Prenatal, perinatal, and neonatal factors in autism, pervasive developmental disorder-not otherwise specified, and the general population. Pediatrics 2001;107(4):E63. [http://dx.doi.org/10.1542/peds.2008-3582] [ Links ]
14. Bailey A, Le Couteur A, Gottesman I, et al. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol Med 1995;25(1):63-78. [http://dx.doi.org/10.1017/S0033291700028099] [ Links ]
15. Buchmayer S, Johansson S, Johansson A, Hultman CM, Sparen P, Cnattingius S. Can association between preterm birth and autism be explained by maternal or neonatal morbidity? Pediatrics 2009;124(5):e817-825. [http://dx.doi. org/10.1542/peds.2008-3582] [ Links ]
16. Libbey JE, Sweeten TL, McMahon WM, Fujinami RS. Autistic disorder and viral infections. J Neurovirol 2005;11(1):1-10. [http://dx.doi.org/10.1080/13550280590900553] [ Links ]
17. Musetti L, Albizzati A, Grioni A, Rossetti M, Saccani M, Musetti C. Autistic disorder associated with congenital HIV infection. Eur Child Adolesc Psychiatry 1993;2(4):221-225. [http://dx.doi.org/10.1007/BF02098581] [ Links ]
18. Pickler L, Elias E. Genetic evaluation of the child with an autism spectrum disorder. Pediatr Ann 2009;38(1):26-29. [http://dx.doi.org/10.3928/00904481-20090101-10] [ Links ]
19. Mubaiwa L, Aziz L, Govender R, Govender V. Pervasive developmental disorders: Clinical characteristics and outcome in African children. Poster presentation, 2012 ICNA Congress, 27 May - 1 June. Dev Med Child Neurol 2012;54(suppl4):151. [ Links ]
20. Zaroff CM, Uhm SY. Prevalence of autism spectrum disorders and influence of country of measurement and ethnicity. Soc Psychiatry Psychiatr Epidemiol 2012;47(3):395-398. [http://dx.doi.org/10.1007/s00127-011-0350-3] [ Links ]
21. Lian WB, Ho SK. Profile of children diagnosed with autistic spectrum disorder managed at a tertiary child development unit. Singapore Med J 2012;53(12):794-800. [ Links ]
22. Laughton B, Springer P, Grove D, et al. Longitudinal developmental profile of children from low socio-economic circumstances in Cape Town, using the 1996 Griffiths Mental Development Scales. South African Journal of Child Health 2010;4(4):106-111. [ Links ]
23. Belhadj A, Mrad R, Halaymem MB. A clinic and a paraclinic study of Tunisian population of children with autism: About 63 cases. Tunis Med 2006;84(12):763-767. [ Links ]
24. South African Medical Research Council, Research Unit for Maternal and Infant Health Care Strategies. PPIP Saving Babies Technical Task Team. Saving Babies 2003 - 2005: Fifth Perinatal Care Survey of South Africa. Pretoria: University of Pretoria, 2005:144. http://www.ppip.co.za (accessed 21 October 2012). [ Links ]
25. Ernhart CB, Morrow-Tlucak M, Sokol RJ, Martier S. Underreporting of alcohol use in pregnancy. Alcohol Clin Exp Res 1988;12(4):506-511. [http://dx.doi.org/10.1111/j.1530-0277.1988.tb00233.x] [ Links ]
26. Hansen RL, Ozonoff S, Krakowiak P, et al. Regression in autism: Prevalence and associated factors in the CHARGE Study. Ambul Pediatr 2008;8(1):25-31. [http://dx.doi.org/10.1016/j.ambp.2007.08.006] [ Links ]
27. Maski KP, Jeste SS, Spence SJ. Common neurological co-morbidities in autism spectrum disorders. Curr Opin Pediatr 2011;23(6):609-615. [http://dx.doi.org/10.1097/MOP.0b013e32834c9282] [ Links ]
28. Fombonne E, Roge B, Claverie J, Courty S, Fremolle J. Microcephaly and macrocephaly in autism. J Autism Dev Disord 1999;29(2):113-119. [ Links ]
29. Barnard-Brak L, Sulak T, Hatz JK. Macrocephaly in children with autism spectrum disorders. Pediatr Neurol 2011;44(2):97-100. [http://dx.doi.org/10.1016/j.pediatrneurol.2010.09.011.0887-8994/$] [ Links ]
30. Matson JL, Shoemaker M. Intellectual disability and its relationship to autism spectrum disorders. Res Dev Disabil 2009;30(6):1107-1114. [http://dx.doi.org/10.1016/j.ridd.2009.06.003] [ Links ]
31. Bolton PF, Carcani-Rathwell I, Hutton J, Goode S, Howlin P, Rutter M. Epilepsy in autism: Features and correlates. Br J Psychiatry 2011;198(4):289-294. [http://dx.doi.org/10.1192/bjp.bp.109.076877] [ Links ]
32. Shevell MI, Majnemer A, Rosenbaum P, Abrahamowicz M. Etiologic yield of autistic spectrum disorders: A prospective study. J Child Neurol 2001;16(7):509-512. [http://dx.doi.org/10.1177/088307380101600710] [ Links ]
33. Battaglia A, Carey JC. Etiologic yield of autistic spectrum disorders: A prospective study. American Journal of Medical Genetics Part C: Seminars in Medical Genetics 2006;142C(1). [http://dx.doi.org/10.1002/ajmg.c.30076] [ Links ]
34. McPartland JC, Reichow B, Volkmar FR. Sensitivity and specificity of proposed DSM-5 diagnostic criteria for autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 2012;51(4):368-383. [http://dx.doi.org/10.1016/j. jaac.2012.01.007] [ Links ]
 Correspondence: P E Springer (springer@sun.ac.za)
Correspondence: P E Springer (springer@sun.ac.za)














