Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.7 n.3 Pretoria Jan. 2013
RESEARCH
Outcomes of neonates with perinatal asphyxia at a tertiary academic hospital in Johannesburg, South Africa
N PadayacheeI; D E BallotII
IMB ChB, DCH (SA)Department of Paediatrics and Child Health, University of the Witwatersrand and Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
IIMB BCh, FCPaed (SA), PhD, Department of Paediatrics and Child Health, University of the Witwatersrand and Charlotte Maxeke Johannesburg Academic Hospital, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Perinatal asphyxia is a significant cause of death and disability.
OBJECTIVE: To determine the outcomes (survival to discharge and morbidity after discharge) of neonates with perinatal asphyxia at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH).
METHODS: This was a descriptive retrospective study. We reviewed information obtained from the computerised neonatal database on neonates born at CMJAH or admitted there within 24 hours of birth between 1 January 2006 and 31 December 2011, with a birth weight of >1 800 g and a 5-minute Apgar score <6.
RESULTS: Four hundred and fifty infants were included in the study; 185 (41.1%) were females, the mean birth weight (± standard deviation) was 3 034.8±484.9 g, and the mean gestational age was 39.1±2.2 weeks. Most of the infants were born at CMJAH (391/450, 86.9%) and by normal vaginal delivery (270/450, 60.0%). The overall survival rate was 86.7% (390/450). Forty-two infants were admitted to the intensive care unit (ICU). The ICU survival rate was 88.1% (37/42). Significant predictors of survival were place of birth (p=0.006), mode of delivery (p=0.007) and bag-mask ventilation at birth (p=0.040). Duration of hospital stay (p=0.000) was significantly longer in survivors than in non-survivors (6.5±6.6 days v. 2.8±9.8 days). The remaining factors, namely gender, antenatal care, chest compressions, diagnosis of meconium aspiration syndrome or persistant pulmonary hypertension, did not differ significantly between the two groups. The rate of perinatal asphyxia (5-minute Apgar score <6) was 4.7/1 000 live births, and there was evidence of hypoxic ischaemic encephalopathy (HIE) in 3.6/1 000 live births. Of the 390 babies discharged from CMJAH, 113 (29.0%) had follow-up records to a mean corrected age of 5.9±5.0 months. The majority (90/113, 79.6%) had normal development.
CONCLUSIONS: (i) The high overall survival and survival after ICU admission provides a benchmark for further care; (ii) obtaining adequate data for long-term follow-up was not possible with the existing resources - surrogate early markers of outcome and/or more resources to ensure accurate follow-up are needed; and (iii) the high incidence of HIE suggests that a therapeutic hypothermia service, including a long-term follow-up component, would be beneicial.
Over 9 million children die each year during the perinatal and neonatal periods, and nearly all of these deaths occur in developing countries.[1] Perinatal asphyxia is a serious clinical problem globally. Every year approximately 4 million babies are born asphyxiated; this results in 1 million deaths and an equal number of serious neurological consequences ranging from cerebral palsy and mental retardation to epilepsy.[2] Perinatal asphyxia is a major factor contributing to perinatal and neonatal mortality, which is an indicator of the social, educational and economic standards of a community.
Perinatal asphyxia is defined as any perinatal insult resulting in suffocation with anoxia and increased carbon dioxide.[2] Severe fetal hypoxia or ischaemia can manifest in the newborn as encephalopathy, and may result in neonatal death or permanent motor and mental disability.[2]
Taking into account that neonatal deaths account for almost 40% of deaths of children under 5, it is apparent that Millennium Developmental Goal 4 (aiming at a two-thirds reduction in under-5 mortality by the year 2015 from a baseline in 1990) can only be met by substantially reducing neonatal deaths. Perinatal asphyxia is the fifth largest cause of under-5 deaths (8.5%) after pneumonia, diarrhoea, neonatal infections and complications of preterm birth.[2]
The death of an infant as a result of perinatal asphyxia is devastating and frequently avoidable. In developed countries with well-functioning health services these deaths are rare and ways to prevent them are widely understood and applied. However, a perinatal audit using the Perinatal Problem Identification Programme (PIPP) (www.ppip.co.za) has identified perinatal asphyxia as a common and important cause of death in South Africa.[3] At Chris Hani Barawagnath Hospital in Gauteng, 20% of all neonatal deaths are due to asphyxia.[4] A group of 25 term asphyxiated infants admitted to the Johannesburg Hospital Neonatal Unit was studied between September 1980 and March 1982. This study showed a mortality rate of 20%, 16% of children were disabled at the 2-year assessment, and 20% were lost to follow-up.[5] In a follow-up retrospective study of 109 term infants with moderate to severe perinatal asphyxia, prognosis was often poor, particularly in patients with seizures, cardiopulmonary signs of asphyxia and multi-organ dysfunction.[6]
The fundamental goal of establishing perinatal audits in areas with high perinatal mortality rates is to reduce the number of perinatal deaths through improvement in the quality of care. Several studies have shown a strong association between the establishment of an effective audit process and improvement of the quality of maternal health services and perinatal mortality rates.[3] Currently there are limited data on perinatal mortality rates, and although available figures are very high, they are likely to underestimate the problem.
The major difficulty in collecting accurate epidemiological data is lack of a common definition of the diagnostic criteria of perinatal asphyxia.[2] The umbilical artery pH that defines asphyxia of a sufficient degree to cause brain injury is unknown. Although the most widely accepted definition is a pH of <7.0, even with this degree of acidosis the likelihood of brain injury is low.[7]
Means of assessment include umbilical pH, 1-hour post-delivery blood gas, Apgar scores, and neurological changes ranging from twitching to hypotonia and seizures. When resources are lacking in developing countries, perinatal asphyxia can be crudely assessed by use of the Apgar score.[8]
Apgar scores at 10 minutes provide useful prognostic data before other evaluations are available for infants. Low Apgar scores at 1, 5 and 10 minutes have been found to be markers of an increased risk of death or chronic motor disability.[9] More scientific methods have been used, but this is not possible in settings where resources are scarce.[10]
The major consequence of perinatal asphyxia is hypoxic ischaemic encephalopathy (HIE). Diagnosis of HIE requires abnormal findings on neurological examination the day after birth. The clinical spectrum of HIE is described as mild, moderate or severe according to the Sarnat stages of HIE. Infants can progress from mild to moderate and/or severe encephalopathy over the 72 hours following the hypoxic-ischaemic insult.[11] The terms 'perinatal asphyxia' and 'HIE' are often inappropriately used to define the same pathology. The problem of benchmarking definitions of HIE in the South African context has been specifically discussed in a recent publication. Horn et al. showed that there is wide variation in the incidence and grade of HIE, depending on which criteria are used.[12]
Until recently, solving the problem of perinatal asphyxia lay mainly in the obstetric realm. Prompt, effective neonatal resuscitation can improve outcome. This has been addressed by the development and implementation of the South African neonatal resuscitation programme,[13] endorsed by the South African Paediatric Association (SAPA).
Cerebral cooling has been shown to significantly improve the outcome for neonates with moderate HIE.[14] In order to effectively implement and monitor such intervention for perinatal asphyxia, it is necessary to have current data on neonates with perinatal asphyxia and those with HIE. Unlike with moderate to severe HIE, the association of perinatal asphyxia with brain damage is not defined, and perinatal asphyxia is not itself an indication for cerebral cooling. The aim of the present study was to review neonates with perinatal asphyxia and determine how many had HIE, the grade of HIE, factors associated with survival at discharge and morbidity on follow-up, and specifically the burden of disease of moderate to severe HIE, as this is the group that requires cooling.
Methods
This was a descriptive retrospective study. The study population included all neonates delivered at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) from 1 January 2006 to 31 December 2011. Babies who were born before arrival (BBA) and those transferred from other hospitals were included if they arrived at CMJAH within 24 hours of birth. The total number of live births at CMJAH and the two major midwife obstetric units affiliated to the hospital was estimated to be 1 310 per month (2011 figures).
Babies were included in the sample if their birth weight was >1 800 g and their 5-minute Apgar score <6.[15] Sarnat and Sarnat classification was used for grading of HIE.[16] Depending on neurobehavioural signs, neonates were divided into stages 1, 2 or 3, which according to the classification correlate with the descriptions of mild, moderate and severe encephalopathy, respectively.[16] Not all babies were assigned a grade of HIE. The recorded discharge diagnoses were reviewed and cases with other evidence of possible encephalopathy (poor suck, lethargy, seizures, decreased tone or lack of responsiveness) recorded on the discharge diagnosis were also noted. These babies were presumed to have HIE, but they could not be graded retrospectively.
Babies with possible causes of low Apgar scores other than perinatal asphyxia, such as chromosomal abnormalities, obvious congenital abnormalities of the central nervous system, spinal muscular atrophy and conditions incompatible with life, were excluded from the study.[6] Those who had meconium aspiration or were subsequently proven to have neonatal sepsis were included.
All attending staff received training according to the SAPA guidelines so that they could start appropriate resuscitation immediately. Babies with grade 2 - 3 HIE were ventilated at the discretion of the attending physician and not routinely, because of resource constraints resulting in limited availability of neonatal intensive care unit (ICU) beds and ventilators. These babies received all other care including supplemental oxygen, fluids, anticonvulsants, nutrition and antibiotics as required. Cerebral cooling was only started at the end of the study period and was done in the ICU. Magnetic resonance imaging was not available during the study period.
Patient information was obtained from the computerised neonatal database at CMJAH, which is kept for clinical audit purposes. Standard information was collected prospectively by attending medical house staff and entered into a Microsoft Access programme by a data capturer. This included demographic data, maternal information, Apgar scores at 1, 5 and 10 minutes, place of delivery, mode of delivery, birth weight, gestational age, need for ventilation after initial resuscitation, presence of seizures, grade of HIE, mortality, duration of hospital stay, cerebral cooling, amplitude electroencephalogram (aEEG) findings, presence of meconium aspiration syndrome (MAS), persistent pulmonary hypertension of the newborn (PPHN), and presence of neonatal sepsis. Only culture-proven sepsis, both early (<72 hours) and late (>72 hours), was considered. The neonatal unit submits mortality data to the PIPP database (http://www.pipp.co.za). Final PIPP diagnosis was reviewed for all deaths.
On discharge of a patient, the parents/caretakers are given an appointment to visit our routine post-discharge neonatal follow-up clinic (NNFU), which is staffed by paediatricians, medical officers, occupational therapists, physiotherapists and speech therapists. Defaulters are contacted once in writing, no further attempt being made to ensure follow-up. Follow-up visits are recorded and stored in the clinic files. NNFU records were reviewed and the findings noted by the attending doctor and the multidisciplinary team were recorded. Outcomes at follow-up were recorded as normal neurological development (age-appropriate milestones achieved, normal vision and hearing), cerebral palsy and developmental delay, microcephaly, cortical blindness, hearing loss or seizures. The outcome at the last clinic attendance was noted for each patient.
Statistical analysis
Data were entered onto an MS-Excel spreadsheet and then imported to the statistical software SPSS version 20 for analysis using standard statistical methods. The analysis ofpatient demographics and baseline outcome variables was summarised using descriptive study methods and expressed as means (± standard deviations (SDs)) or medians (ranges) for continuous variables and frequencies and percentages for categorical variables. Survivors and non-survivors were compared. Categorical data were compared using chi-square analysis and continuous data using unpaired t-tests (as the distribution was normal).
Ethics approval
The study was approved by the Committee for Research on Human Subjects of the University of Witwatersrand (clearance certiicate number M120447).
Results
A total of 470 babies were eligible for inclusion into the study; however, 7 were excluded because there were no clinical data available at all, and 13 owing to major congenital abnormalities. The final sample therefore consisted of a total of 450 babies, 185 females (41.1%) and 260 males (57.8%). The mean ±SD maternal age was 25.6±6.1 years and mean parity was 1 (interquartile range 1 - 9). Birth weights ranged from 1 800 to 4 596 g (mean ±SD 3 034.8±484.9 g), and the mean ±SD gestational age was 39.1±2.2 weeks. Clinical and demographic characteristics are set out in Table 1.
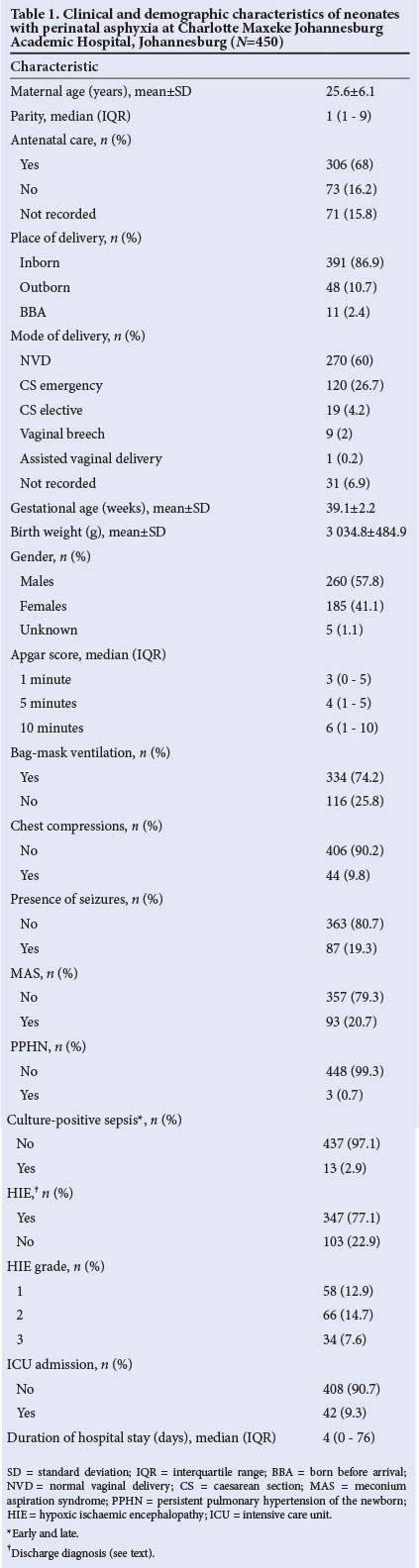
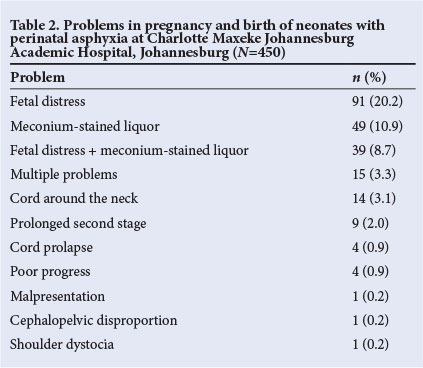
The majority of the babies were born at CMJAH (391/450, 86.9%) and by normal vaginal delivery (270/450, 60.0%). Problems in pregnancy were documented in 228/450 cases (50.7%) (Table 2), fetal distress being the most common (91/450, 20.2%).
A total of 346 babies (76.9%) had evidence of HIE, but only 158 (45.7%) of these had a grade of HIE recorded The rate of perinatal asphyxia (5-minute Apgar score <6) was 4.68/1 000 live births, and there was evidence of HIE in 3.6/1 000 live births.
ICU admissions
Forty-two babies were admitted to the ICU, 15 females and 27 males. Of these 15 (35.7%) had MAS, 9 (21.4%) had hyaline membrane disease (HMD) and 3 (7.1%) had PPHN. The ICU survival rate was 88.1% (37/42). Two of the babies received cerebral cooling after perinatal asphyxia. They were monitored in the neonatal ICU and both survived.
Mortality
The overall survival rate was 86.7% (390/450). The causes of death according to the PIPP classification were as follows: perinatal asphyxia 53/60 (88.3%), meconium aspiration and perinatal asphyxia 3/60 (5.0%), HMD 1/60 (1.7%), group B streptococcal infection 2/60 (3.3%) and hypovolaemic shock 1/60 (1.7%). Significantly, more babies who died compared with those who survived had evidence of HIE (Table 3).
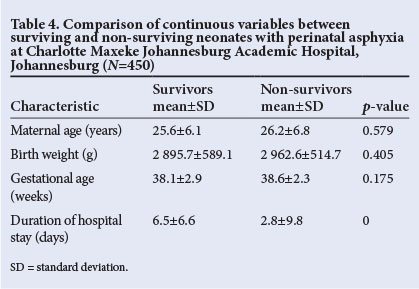
Comparison between survivors and non-survivors
Various factors were compared between the babies who survived and those who died (Tables 3 and 4). Place of birth (p=0.006), mode of delivery (p=0.007) and bag-mask ventilation at birth (p=0.040) were all significantly associated with survival. The duration of stay (p=0.000) was significantly longer in survivors. The remaining factors, namely gender, antenatal care, chest compressions, diagnosis of meconium aspiration syndrome and persistent pulmonary hypertension of the newborn, did not differ significantly between the two groups.
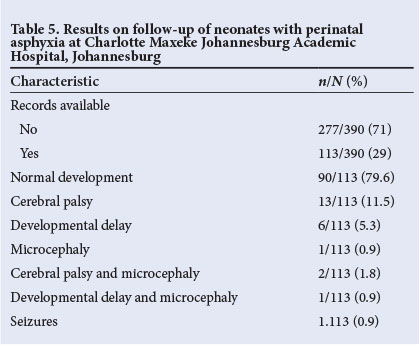
Follow-up
Of the 390 babies discharged from CMJAH, 113 (29.0%) had follow-up records to a mean ±SD corrected age of 5.9±5.0 months. The majority (90/113, 79.6%) had normal development. Details of the disabilities are shown in Table 5.
Discussion
This review shows that perinatal asphyxia remains a common problem at CMJAH, with approximately 6 admissions every month. In-hospital mortality was low (60/450, 13.3%), with the burden anticipated to be in the disabled survivors. Our rates of perinatal asphyxia and HIE were similar to those found by Horn et al.[12]
The 5-minute Apgar score is a poor indication of cerebral injury. In this study, 103 babies (22.9%) had no evidence of HIE, although 347 (77.1%) had signs of neurological compromise recorded. Attending staff do not routinely allocate a grade of HIE, and only 158 babies had a grade recorded. This is a very important omission, as it is a difficult thing to decide retrospectively. Possible reasons for the lack of proper grading or a detailed neurological examination may be related to challenges in a busy resource-constrained setting, lack of continuity of care (as different healthcare workers review patients daily), or healthcare workers not having been adequately trained on the criteria to examine for and the grading to allocate.
There are few population-based studies of HIE in sub-Saharan Africa, and the published criteria that are used to define and grade HIE are too variable for meaningful comparisons between studies and populations. Horn et al.[12] discuss the difficulties in consensus definitions and criteria of HIE. The data show that there is a wide variation in the incidence and grade of HIE, depending on which criteria are used. A more refined method of classifying perinatal asphyxia than the 5-minute Apgar score is required, possibly the TOBY[14] or CoolCap[17] definitions; however, these require special investigations. There is a need to encourage staff to assign an HIE grade accurately. The need for resuscitation at birth predicted outcome and could be included in the definition of perinatal asphyxia in resource-poor settings, where there is no means of arterial blood gas measurement or aEEG available.
Our busy resource-constrained setting, where only 74.2% of infants born with a 5-minute Apgar score <6 receive bag-mask resuscitation, presents major challenges. Before a cooling programme is implemented, it is essential to ensure adequate neonatal resuscitation and strict HIE grading of all asphyxiated neonates.
Our follow-up rate is unacceptably low at only 29.0% (113/390), and it was disappointing that the 2 babies who had received cerebral cooling were not brought for follow-up. Failure to attend for follow-up may be due to socio-economic factors and the low level of education of our patients' parents/guardians. It is possible that some asphyxiated babies died after discharge, or that disabled children are kept at home without access to healthcare. It is therefore not possible to report rates of post-discharge disability or mortality accurately. Of the 113 patients with follow-up data, 24 (21.2%) had disability.
The study results show that predictors of survival were mode of delivery, place of birth and resuscitation at birth. Elective caesarean section was associated with improved outcomes. Unexpectedly, all babies with vaginal breech deliveries survived. A study has shown that in fetal breech presentation, neonatal outcome was better with planned caesarean section than vaginal breech delivery.[18] In contrast, a study from Europe showed that neonatal outcome after planned vaginal breech delivery did not differ from outcome after elective caesarean section.[19] However, we do not know whether our breech deliveries were planned. All the babies BBA survived. It is possible that more severely asphyxiated babies died at home or were dead on arrival at the hospital, but data relating to that information were beyond the scope of this study. Duration of hospital stay was shorter for babies who died than for those who survived, indicating that their condition was very severe and resulted in early death.
Study limitations
This was a retrospective study that relied on data from attending staff, with possible inaccuracies and loss of data. This study describes the incidence of perinatal asphyxia as defined by a 5-minute Apgar score <6, and there are insufficient data to comment on the incidence of moderate to severe HIE or on morbidity after discharge. Lack of a clear deinition of HIE was a further limitation. A prospective follow-up study of babies who sustain perinatal asphyxia is warranted.
Conclusion
The study confirms that perinatal asphyxia remains a significant problem at CMJAH. The high overall survival and survival after ICU admission provide a benchmark for further care. There is a need to obtain adequate data for long-term follow-up, as this was not possible with the existing resources. Further research is required to establish consensus deinitions that can be used for meaningful population studies and benchmarking of HIE. More resources to ensure accurate follow-up are needed, and the high incidence of HIE suggests that a therapeutic hypothermia service including a long-term follow-up component would be beneicial.
References
1. Hoque M, Haaq S, Islam R. Causes of neonatal admissions and deaths at a rural hospital in KwaZulu-Natal, South Africa. South African Journal of Epidemiology and Infection 2011;26(1):26-29. [ Links ]
2. Lawn JE, Manandhar A, Haws RA, Darmstadt GL. Reducing one million child deaths from birth asphyxia - a survey of health systems gaps and priorities. Health Res Policy Syst 2007;5(1):4. [http://dx.doi.org/10.1186/1478-4505-5-4] [ Links ]
3. Pattinson RC, ed. Saving Babies 2008-2009: Seventh Report on Perinatal Care in South Africa. http://www.ppip.co.za/downloads/Saving%20Babies%202008-9.pdf (accessed 21 June 2013). [ Links ]
4. Cooper PA, Patrick K. Analysis of term asphyxiated infants at Baragwanath Hospital. Proceedings of the Eleventh Conference on Priorities in Perinatal Care in South Africa. Johannesburg: Department of Paediatrics, University of the Witwatersrand, 1992. [ Links ]
5. Wild C, Rothberg AD, Cooper PA, Thompson PD, Cohn R. Outcome for premature infants versus full term asphyxia. Proceedings of the Third Conference on Priorities in Perinatal Care in South Africa. Johannesburg: Department of Paediatrics, University of the Witwatersrand, 1984. [ Links ]
6. Scher LG. Outcome following asphyxial insult in term neonates. MMed (Paed) thesis, University of the Witwatersrand, Johannesburg, 2000. [ Links ]
7. Thornberg E, Thiringer K, Odeback A, Milsom I. Birth asphyxia: Incidence, clinical course and outcome in a Swedish population. Acta Paediatr 1995;84(8):927-932. [http://dx.doi.org/10.1111/j.1651-2227.1995.tb13794.x] [ Links ]
8. World Health Organization. Birth Asphyxia - Summary of the previous meeting and protocol overview. 11 June 2007. http://www.curoservice.com/health_professionals/news/pdf/10-09-2007_birth_asphyxia02.pdf (accessed 5 August 2013). [ Links ]
9. Nelson KB, Ellenberg JH. Apgar scores as predictors of chronic neurologic disability. Pediatrics 1981;68(1):36-44. [ Links ]
10. Gregersen NE, Ballot DE, Guidozzi F, Cooper PA. Birth asphyxia, presenting a case for 'a stitch in time. S Afr Med J 1999;89(3):326-332. [ Links ]
11. Robertson CM, Perlman M. Follow-up of the term infant after hypoxic-ischemic encephalopathy. Paediatr Child Health 2006;11(5):278-282. [ Links ]
12. Horn AR, Swingler GH, Myer L, et al. Defining hypoxic ischemic encephalopathy in newborn infants: Benchmarking in a South African population. J Perinat Med 2013;41(2):211-217. [http://dx.doi.org/10.1515/jpm-2012-0107] [ Links ]
13. Ballot D, Adhikari M, Bolton K, et al. South African Handbook of Rescusitation of the Newborn. Johannesburg: South African Paediatric Association, 2004. [ Links ]
14. Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361(14):1349-1358. [http://dx.doi.org/10.1056/NEJMoa0900854] [ Links ]
15. Misra PK, Srivastava N, Malik GK, et al. Outcome in relation to Apgar score in term neonates. Indian Pediatr 1994;31(10):1215-1218. [ Links ]
16. Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: A clinical and electroencephalographic study. Arch Neurol 1976;33(10):696-705. [http://dx.doi.org/10.1001/archneur.1976.00500100030012] [ Links ]
17. Jacobs S, Hunt R, Tarnow-Mordi W, Inder TP, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2007;(4): CD003311. [http://dx.doi.org/10.1002/14651858.CD003311.pub2] [ Links ]
18. Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR, Term Breech Trial Collaborative Group. Planned caesarean section versus planned vaginal birth for breech presentation at term: A randomised multicentre trial. Lancet 2000;356(9239):1375-1383. [http://dx.doi.org/10.1016/S0140-6736(00)02840-3] [ Links ]
19. Goffmet F, Carayol M, Foidart J-M, et al. Is planned vaginal delivery for breech presentation at term still an option? Results of an observational prospective survey in France and Belgium. Am J Obstet Gynecol 2006;194(4):1002-1011. [http://dx.doi.org/10.1016/j.ajog.2005.10.817] [ Links ]
 Correspondence: N Padayachee (natasha.padayachee@gmail.com)
Correspondence: N Padayachee (natasha.padayachee@gmail.com)














