Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Child Health
versión On-line ISSN 1999-7671
versión impresa ISSN 1994-3032
S. Afr. j. child health vol.7 no.2 Pretoria may. 2013
CASE REPORT
Fetal valproate syndrome in a 14-month-old child: A case report
A AbbasI; U FirdausII
IMD, MRCPCH. Department of Pediatrics, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, Uttar Pradesh, India
IIMD. Department of Pediatrics, Jawaharlal Nehru Medical College, Aligarh Muslim University, Aligarh, Uttar Pradesh, India
ABSTRACT
Anti-epileptic drugs administered during pregnancy can cause structural defects in the growing fetus and result in adverse neuro-developmental outcome later in life. Fetal valproate syndrome (FVS) results from teratogenic effects of valproic acid exposure in the prenatal period. It is characterised by a distinctive dysmorphic facies and a cluster of minor and major anomalies, as well as developmental and behavioural abnormalities. We describe a 14-month-old boy with the typical dysmorphic facies and other congenital abnormalities characteristic of FVS. There was a history of maternal intake of sodium valproate during pregnancy. The mechanism of teratogenicity and methods for prevention are discussed.
Valproic acid (VPA) or 2-propyl-pentanoic acid was first introduced for use as an anti-epileptic drug (AED) in 1964. It is still commonly used for the management of epilepsy and certain mood disorders, despite increased awareness of its adverse effects, particularly when taken during pregnancy. The teratogenic effects of VPA were first recognised in 1980.[1] Since then many potential teratogenic and dysmorphogenic effects have been reported. More recently, there has been a better recognition of developmental and behavioural abnormalities associated with fetal valproate syndrome (FVS). We report a case of FVS in a 14-month-old boy whose epileptic mother had taken sodium valproate during her pregnancy.
Case report
A 14-month-old boy with cough and a fever was brought to our outpatient department. He was the second child born of a non-consanguineous marriage. His mother had been taking VPA for epilepsy for the past 3 years. She had been initiated on treatment for postpartum eclampsia after the birth of her first child, and was on a dose of 1 200 mg/day throughout her pregnancy. The infant was born at term at a peripheral hospital by the vaginal route. His birth weight was 2 300 g. He cried immediately after birth, and the postnatal period was uneventful. The intrauterine growth retardation and the possibility that the AED might have teratogenic effects were not considered by the healthcare providers at the peripheral hospital, so no specific tests were done in the antenatal period. Soon after the baby's birth the parents noted his clubbed left hand and sought treatment. An orthopaedic practitioner applied repeated plaster casts, but the deformity did not improve.
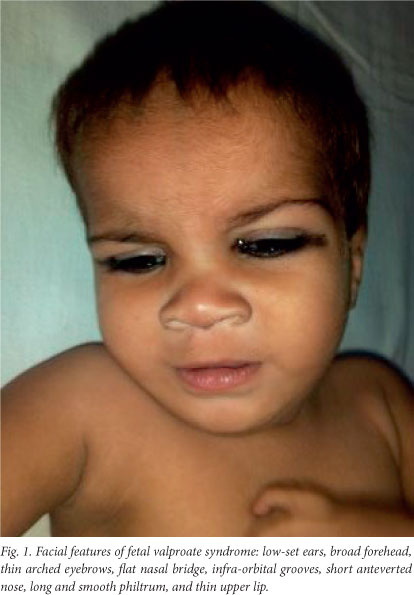
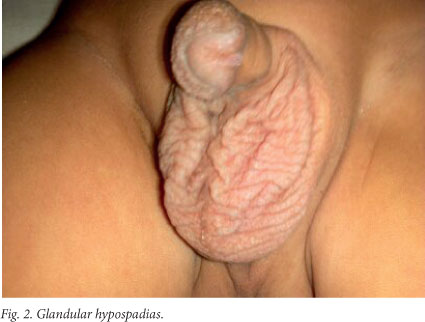
On examination at our hospital the patient's weight was 6.5 kg, his length was 75.5 cm (below the 5th percentile for age and sex), and his head circumference was 44.6 cm (just above the 3rd percentile for age and sex). He had dysmorphic features in the form of low-set ears, a broad and depressed nasal bridge, a long philtrum, an upturned nose, a thin upper lip and a thick lower lip, with thin vermillion borders, and a small mouth (Fig. 1). Broad hands and feet, the left radial club hand, loose skin and glandular hypospadias (Fig. 2) were other notable features. He also showed global developmental delay. He was able to sit without support but was not able to stand, was able to transfer objects between his hands, and had an unrefined pincer grasp. He spoke in monosyllables and responded when his name was spoken, but showed no stranger anxiety.
Two-dimensional echocardiography showed no cardiac abnormality. An ultrasound scan of the abdomen was also normal. A radiograph of the dorsolumbar spine revealed an unfused spinous process of the L5 vertebra. A radiograph of the skull was normal. In view of the history of maternal intake of VPA during pregnancy and the typical dysmorphic features, a diagnosis of FVS was made.
Discussion
None of the currently available AEDs are completely safe during pregnancy, but VPA appears to be the most teratogenic. In approximately 1 in 250 pregnancies the fetus is known to be exposed to AEDs, and a significant proportion ofthese are exposed to VPA.[2] The overall risk of major congenital malformation (MCM) in patients receiving AEDs during pregnancy is 4.2%. The MCM rate is higher for polytherapy than for monotherapy, and polytherapy regimens containing valproate result in significantly more MCMs than those not containing valproate. For monotherapy exposure, valproate has a higher MCM rate than any other AED if taken in a dose exceeding 1 000 mg per day.[3]
There have been no reports of an increased risk of maternal complications during pregnancies exposed to VPA, or of increased rates of instrumental deliveries, birth asphyxia or stillbirths in FVS. The majority of affected babies are of average birth weight, although 10% are small for gestational age and 11 - 17% have a large birth weight of over 4 000 g.[4] Our patient had a low birth weight and no perinatal complications.
The dysmorphic features described in FVS consist of a prominent metopic ridge, thin arched eyebrows with medial deficiency, epicanthic folds, infra-orbital grooves, a broad nasal bridge, a short anteverted nose and a smooth, long philtrum with a thin upper lip.[5] However, it is not the individual dysmorphic features but the complete facial phenotype that provides clues to the presence of the condition. These features are best appreciated in infancy, as they become less obvious with age. Our patient had the typical dysmorphic facies for FVS.
There is a 6 - 9% risk of congenital malformations in infants exposed to VPA prenatally, compared with 2 - 3% in the general population.[3] Babies of mothers exposed to VPA have a 10 times increased risk of neural tube defects (NTDs). The defects are mostly in the lower dorsal and sacral region and are skin covered. The level of maternal alpha-fetoprotein (AFP) may therefore not rise in the presence of NTD. This makes targeted ultrasound of the caudal spine more important than raised AFP levels in the evaluation of valproate-exposed fetuses. Our patient had skin-covered spina bifida involving the L5 vertebra. In his review of the literature on children with FVS from 1978 to 2000, Kozma found that the majority of patients had musculoskeletal abnormalities. Other deformities in decreasing order of frequency were skin defects, cardiovascular abnormalities, genital and pulmonary abnormalities and neural tube defects.[6] Our patient, however, had no clinical evidence of any congenital heart disease, and this was later confirmed by a 2D echocardiogram.
The risk of oral clefts seems to increase only when VPA is used in combination with other AEDs.[7] This is consistent with our case, in which no oral cleft was present. The risk of a limb abnormality from VPA exposure has been estimated to be about 0.42%. This may include radial ray defects, split hand, post-axial polydactyly and pre-axial polydactyly.[8] Our patient had a left radial club hand, but no other limb anomaly was present. Hypospadias and undescended testes are seen relatively frequently with VPA exposure. Renal abnormalities, including renal hypoplasia, hydronephrosis and duplication of the calyceal system, have been reported less frequently.[9] Our patient had a glandular hypospadias, but both testes were descended and no renal abnormalities were detected on the ultrasound scan.
Global developmental delay is present only in severely affected cases. In general, the most frequently affected developmental aspect is speech and language.[10] Our patient showed evidence of global developmental delay. However, he had no features suggestive of autism, Asperger's syndrome or autistic spectrum disorder, although these are frequently associated with FVS.
There is still uncertainty about the mechanism of teratogenicity of VPA. It has been suggested that the accumulated epoxides may act as teratogen. Epoxide hydrolase is the enzyme involved in VPA metabolism, the inhibition of which results in accumulation of epoxides. Alteration in intracellular pH, interference with embryonic lipid and zinc metabolism are other proposed causes for teratogenicity.[11]
There is often a wide variation in the clinical presentation of the condition, even between siblings, which makes the diagnosis difficult. This variation in presentation is attributed to a number of factors such as such as maternal seizures during pregnancy, folic acid intake, dose and timing of exposure of VPA, parental factors such as IQ and socio-economic status, and genetic susceptibility.
Management of FVS includes surgical management of the malformations wherever indicated and feasible. Early speech therapy and physiotherapy for patients with motor developmental delay is another cornerstone in the treatment. Extra help at school may be needed for a large proportion of children with learning disability.
VPA is a widely used AED, particularly in resource-poor countries, and its efficacy as a broad-spectrum antiepileptic is undisputed. Nevertheless, a balance between the therapeutic effects of this drug and its teratogenic effects is critical in the management of women with epilepsy. In focal-onset epilepsy, alternatives such as carbamazepine are effective and should be used in view of their better safety profile during pregnancy. An attempt should be made to reduce the dose of VPA below 1 000 mg/day, as the risk of teratogenicity is much higher above this dose. Also, women who take more than one medication for control of their epilepsy should be advised to change to monotherapy if possible, as polytherapy is reported to be more harmful. In addition, high-dose folic acid (4 mg/day) should be given to all pregnant women on anti-epileptic treatment, starting at least 6 weeks before conception and continuing through the first trimester.[12] Folic acid supplements are thought to be protective against malformations, in particular against NTDs.
Reference
1. Dalens B, Raynaud EJ, Gaulme J. Teratogenicity of valproic acid. J Pediatr 1980;97(2):332-333. [http://dx.doi.org/10.1016/S0022-3476(80)80517-8] [ Links ]
2. Lindhout D, Omtzigt JG. Pregnancy and the risk of teratogenicity. Epilepsia 1992;33:S41-S48. [http://dx.doi.org/10.1111/j.1528-1157.1992.tb06226.x] [ Links ]
3. Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: A prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry 2006;77(2):193-198. [http://dx.doi.org/10.1136/jnnp.2005.074203] [ Links ]
4. Kini U, Adab N, Vinten J, Fryer A, Clayton-Smith J; Liverpool and Manchester Neurodevelopmental Study Group. Dysmorphic features: An important clue to the diagnosis and severity of fetal anticonvulsant syndromes. Arch Dis Child Fetal Neonatal Ed 2006;91(2):90-95. [http://dx.doi.org/10.1136/adc.2004.067421] [ Links ]
5. Winter RM, Donnai D, Burn J, Tucker SM. Fetal valproate syndrome: Is there a recognisable phenotype? J Med Genet 1987;24(11):692-695. [http://dx.doi.org/10.1136/jmg.24.11.692] [ Links ]
6. Kozma C. Valproic acid embryopathy: Report of two siblings with further expansion of the phenotypic abnormalities and a review of the literature. Am J Med Genet 2001;98(2):168-175. [http://dx.doi.org/10.1002/1096-8628(20010115)98:2<168::AID-AJMG1026>3.0.CO;2-O] [ Links ]
7. Martinez-Frias ML. Clinical manifestation of prenatal exposure to valproic acid using case reports and epidemiologic information. Am J Med Genet 1990;37(2):277-282. [http://dx.doi.org/10.1002/ajmg.1320370224] [ Links ]
8. Rodriguez-Pinilla E, Arroyo I, Fondevilla J, Garcia MJ, Martinez-Frias ML. Prenatal exposure to valproic acid during pregnancy and limb deficiencies: A case-control study. Am J Med Genet 2000;90(5):376-381. [http://dx.doi.org/10.1002/(SICI)1096-8628(20000228)90:5<376::AID-AJMG6>3.3.CO;2-M] [ Links ]
9. Jager-Roman E, Deichl A, Jakob S. Fetal growth, major malformations and minor anomalies in infants born to women receiving valproic acid. J Pediatr 1986;108(6):1997-1004. [http://dx.doi.org/10.1016/S0022-3476(86)80949-0] [ Links ]
10. Adab N, Kini U, Vinten J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry 2004;75(11):1575-1583. [http://dx.doi.org/10.1136/jnnp.2003.029132] [ Links ]
11. Hurd RW, Wilder BJ, Van Rinsvelt HA. Valproate, birth defects and zinc. Lancet 1983;1(8317):181-181. [http://dx.doi.org/10.1016/S0140-6736(83)92775-7] [ Links ]
12. Crawford P, Appleton R, Betts T, et al. the Women with Epilepsy Guidelines Development Group. Best practice guidelines for the management of women with epilepsy. Seizure 1999;8(4):201-217. [http://dx.doi.org/10.1053/seiz.1999.0295] [ Links ]















