Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.7 n.1 Pretoria Jan. 2013
RESEARCH
Neutrophil CD64 has a high negative predictive value for exclusion of neonatal sepsis
M B DhlaminiI; M S SuchardII; T M WiggillIII; O O FadahunIV; D E BallotV
IMB ChB, FCPath (SA) Haem, MMed (Haem); Department of Molecular Medicine and Haematology, National Health Laboratory Service and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg
IIBSc, MB BCh, FCPath (SA) Clin, MMed (Clin), DTM&H; Department of Molecular Medicine and Haematology, National Health Laboratory Service and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg
IIIMB BCh, MMed (Haem); Department of Molecular Medicine and Haematology, National Health Laboratory Service and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg
IVMB BS, MSc (Med) Epidemiol; Centre for Health Science Education, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg
VMB BCh, FC Paed (SA), PhD; Neonatal Unit, Department of Paediatrics and Child Health, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences, University of the Witwatersrand, Johannesburg
ABSTRACT
BACKGROUND AND AIM: Neonatal sepsis is a significant contributor to morbidity and mortality globally. Blood culture is the most reliable method for detection of bacterial infection. However, its sensitivity is low and its use in the diagnosis of bacteraemia is fraught with difficulties. CD64 antigen is up-regulated in neutrophils only when they are activated, and has been shown to be a potential biomarker for infection. The primary objective of this study was quantitation of neutrophil CD64 by flow cytometry in neonates with signs and symptoms suggestive of sepsis/infection in the first 4 weeks of life.
METHODS AND RESULTS: In this prospective observational study, patients were classified into categories of infection, namely definite, probable and possible. Of 76 neonates, 1 had definite infection, 5 probable infection, 30 possible infection, and 32 no infection. The neutrophil CD64 index at a cut-off value of 1.8 had a high negative predictive value (95.2%) in ruling out combined probable and definite infection.
CONCLUSIONS: We recommend inclusion of the neutrophil CD64 index into the diagnostic algorithm for neonatal sepsis, as it has a high negative predictive value and can be used to rule out infection. As the positive predictive value of the index was low in confirming infection, it should be used as a screening rather than a confirmatory test.
Neonatal sepsis remains a global health problem owing to its significant contribution to morbidity and mortality.[1-3] Its early diagnosis presents a clinical dilemma because of varied and nonspecific clinical presentation.[1,4-6] Microbiological cultures are currently used as the gold standard for diagnosis of infection/ sepsis and help in decision-making, especially in choosing the appropriate antibiotics.[2,7,8] However, the method is fraught with difficulties, including delay in final culture results (2 - 4 days), negative culture results in patients exposed to antibiotics, and timing and technique of specimen collection.[2-8] Despite being widely acknowledged as lacking specificity and sensitivity, the white cell count (WCC), platelet count (Plt) and white cell differential count (Diff) are still used for the screening of neonates with sepsis.[2,9] Acute-phase reactants are also frequently used in predicting neonatal sepsis.[5,6,10] The most extensively used acute-phase reactant is C-reactive protein (CRP).[11] In a recent meta-analysis the sensitivity of this test was estimated to be 80%, but the specificity was only 60 - 80%.[9] Several studies agree that serial measurements of CRP can guide the duration of antibiotic treatment in neonates managed for suspected sepsis before the final blood culture results are available.[3,6,11,12] One of the newer acute-phase markers of infection is procalcitonin (PCT), the prohormone of calcitonin, which occurs in very low concentrations in the serum of healthy people.[5] PCT is claimed to be more specific for bacterial infections than viral infections, but it is not universally accepted as an improved diagnostic assay of infection.[13]
Recently various investigators have focused their research on the diagnostic and clinical utility of neutrophil CD64 as a marker of sepsis or systemic infection in neonates[1,4,7,14] and adults.[1315,16] CD64 is a 72 kDa glycoprotein, known as FC gamma receptor I (FcyRI), that binds immunoglobulin G with high affinity. '171 CD64 is constitutively expressed on monocytes and various types of macrophages, but only to a very low extent on resting neutrophils.[9,17] During neutrophil activation, under the influence of inflammatory cytokines (interleukin-12, interferon gamma and granulocyte colony-stimulating factor), there is upregulation of neutrophil CD64.[13] Multiple studies agree that neutrophil CD64 has high diagnostic specificity and sensitivity.[13,18-20] In the light of the above information, we prospectively evaluated the usefulness of neutrophil CD64 expression in diagnosing neonatal infection. The main objective of the study was quantitation of neutrophil CD64 by flow cytometry in all neonates with signs and symptoms suggestive of sepsis/infection within the first 4 weeks of life.
Material and methods
Research design and site
This prospective observational study was conducted at three hospitals in Johannesburg, South Africa, namely Rahima Moosa Mother and Child, Chris Hani Baragwanath and Charlotte Maxeke Johannesburg Academic. Consecutive neonates with suspected infection or sepsis were enrolled from June 2009 to July 2010.
Ethical clearance was given by the Human Research Ethics Committee of the University of the Witwatersrand, Johannesburg, under protocol number M081106. Written informed consent was given by the parent/s of the neonates.
Inclusion criteria and study definitions
All neonates (days 1 - 28) suspected of having infection/sepsis by their treating clinician were eligible for enrolment in the study. Infection was suspected when nonspecific signs and symptoms including respiratory distress, tachypnoea, tachycardia, hypoglycaemia and hyperglycaemia were present, or when there were maternal risk factors.
Neonates with severe congenital or chromosomal abnormalities were excluded from the study. The septic work-up was done at the clinician's discretion, but the full blood count (FBC), Diff (not performed for every patient), CRP level and blood culture were all recorded for the purposes of the study. The CRP level was measured on the second day (after 24 - 48 hours of suspicion of sepsis), while other tests were done on the first day of suspicion of sepsis.
Neonates who had been on antibiotics for 48 hours or more, but clinically appeared septic and needed re-evaluation (another septic work-up), were also included in the study.
Categories of infection
Categories of infection were defined using previously published criteria:[6]
- Contamination. Positive microbiological cultures with less than three signs and symptoms of infection and normal WCC, Dif, Plt and CRP.
- Possible infection. One or more of abnormal Plt, WCC, Diff or CRP with negative microbiological cultures and less than three signs or symptoms of infection.
- Probable infection. One or more of abnormal Plt, WCC, CRP or Dif with negative microbiological cultures with three or more signs or symptoms of infection.
- Definite infection. Positive microbiological cultures with any clinical signs or symptoms of infection and one or more of abnormal WCC, Diff, Plt or CRP.
- No infection. Normal CRP, WCC, Diff and Plt with negative microbiological cultures and less than three symptoms or signs of infection.
- Unclassified. Do not fit into any of the other categories.
In this study, however, Dif was not used to categorise infection, as this test was not done in the majority of the patients.
Sample collection and storage
The attending paediatrician collected an additional tube of 0.3 ml peripheral blood (purple top with ethylenediaminetetra-acetic acid (EDTA), anticoagulant) for the purposes of this study concurrently with the other septic work-up blood investigations. This blood was stored at 4°C until informed consent was obtained from the parents for participation in the study. If consent was not obtained, the blood was discarded. Samples were processed within 48 hours of the blood being drawn, as per the manufacturer's guidelines.
Clinicians were unaware of the neutrophil CD64 results.
Collation of clinical and laboratory data
The clinical history was retrieved from the hospital medical files, both neonatal and maternal. Available laboratory results were retrieved from the laboratory information system: FBC, Diff and blood culture at the time of the septic workup and CRP the next day.
Quantitation of neutrophil/polymorphonuclear cell (PMN) CD64
The Leuko64 kit (Trillium Diagnostics, LLC, ME, USA) is composed of antibodies with specificities to CD64 (clones 22 and 32.2, both fluorescein isothiocyanate conjugated) and to CD163 (clone Mac2-148, phycoerythrin conjugated), and a fluorescent bead suspension. The sample preparation and flow cytometry setup was based on the manufacturer's instructions.[21] Briefly, 50 µl of whole blood was incubated for 15 minutes in the dark at room temperature with a mixture of the monoclonal antibodies followed by red cell lysis with ammonium chloride-based red cell lysis solution. Fluorescent beads were then added and low cytometry analysis performed on the LSRII flow cytometer (Becton-Dickson, San Jose, CA) using FACSDIVA software. The CD64 index calculation was performed by Leuko64 QuantiCALC software (Trillium Diagnostics). An internal negative control was provided by the automated measurement of the lymphocyte CD64 index, and an internal positive control was provided by automated measurement of the monocyte CD64 index.
Statistical analysis
Stata 10.0 software was used to analyse data from a total of 76 neonates. Normality of data was tested using the Shapiro-Wilk test. Birth weight of participants, PMN CD64 and WCC showed a positively skewed distribution. Descriptive statistics for categorical (frequency and percentages) and continuous variables (median) were reported. Logistic regression models were used to determine the association between selected neonatal factors (gender and birth weight) and infection. The logistic model considered infection (blood culture positive) as the outcome variable of interest and neonatal factors as independent factors. Logistic regression models were also used to determine the association between selected neonatal factors (gender, gestational age and birth weight) and PMN CD64. Abnormal PMN CD64, i.e. >1.8,[21] was considered as the outcome of interest with the selected neonatal factors as independent variables. Receiver operating characteristic (ROC) curves were generated for PMN CD64 at various cut-offs by plotting sensitivity versus 1-specificity (specificity is the true-negative rate, that is the probability that a test result will be negative when the disease is not present; 1-specificity is the false-positive rate, the probability of a positive test result given in the absence of the disease). PMN CD64 indices of >1.8, >1.6 and >2.0 were considered as the outcome of interest. p-values were reported as statistically significant if <0.05.
Results
Study population
The total number of neonates screened was 141, and 76 participants met the inclusion criteria.
Patient characteristics
According to the clinical findings and blood culture results, patients were classified into categories of infection as defined in 'Categories of infection' above. The number of participants finally assigned to each category was as follows: definite infection (1), probable infection (5), possible infection (30), no infection (32), unclassified (8). Table 1 shows the demographic data.
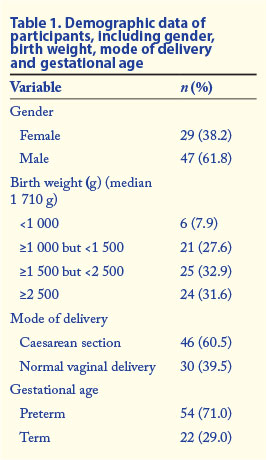
The neonate who had definite infection (positive blood culture) was female, preterm, and of very low birth weight (890 g). She was delivered by caesarean section and was 4 days old at the time of enrolment. Blood culture was positive for Gram-negative bacilli (Klebsiella pneumoniae, extended-spectrum beta-lactamase producer). Her CRP level was 29.0 mg/l and her PMN CD64 index 3.70.
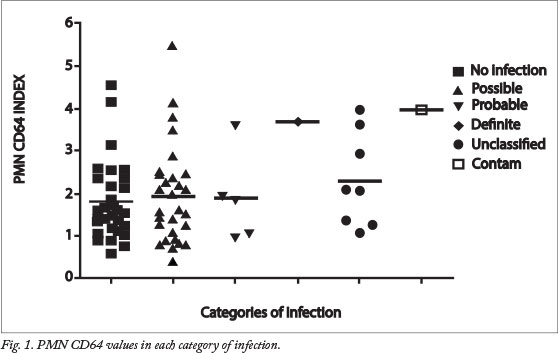
The most frequent clinical sign in the study was respiratory distress (92.1%, 70/76), followed by tachypnoea (19.7%, 15/76). Other signs and symptoms included hypoglycaemia (6.6%, 5/76), tachycardia (3.9%, 3/76), hypothermia (2.6%, 2/76), failure to wean from oxygen (2.6%, 2/76) and hyperglycaemia (1.3%, 1/76). Fig. 1 illustrates PMN CD64 values in each category of infection, and Table 2 summarises the participants' blood results. According to the PMN CD64 index classified as normal (<1.8) versus high (>1.8), PMN CD64 was not statistically associated with gender, birth weight or gestational age.
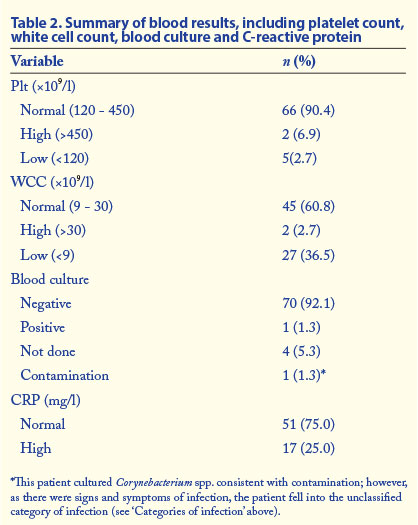
Receiver operating characteristic analysis
The ROC curve is a graphical presentation of trade-off between sensitivity and specificity. The greater the area under the ROC curve (AUC), the more discriminatory the test is. The AUC measures the overall ability of the test (continuous variable) to discriminate between individuals with and without disease. In this study, ROC curves were done to compare PMN CD64 with the current gold standard test for diagnosis of infection and sepsis (blood culture). In addition, the performance of the PMN CD64 was also compared with our infection categories, using them as the gold standard. ROC curves were done at three different cut-off values of PMN CD64 index (1.6, 1.8 and 2.0). Fig. 2 shows the ROC curve of PMN CD64 at a cut-off value of 1.8 versus definite and probable infection.
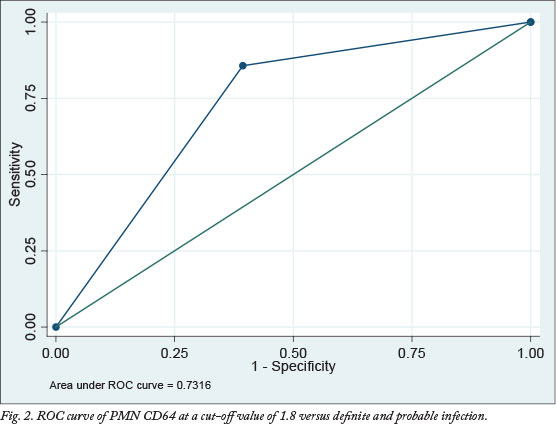
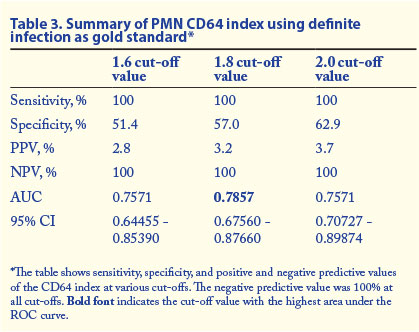
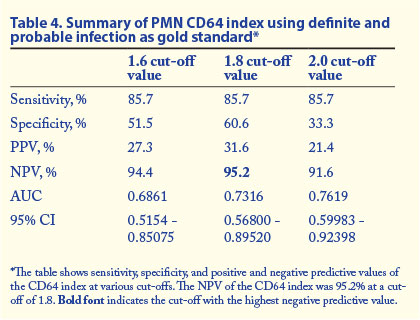
Tables 3 and 4 show the summary of the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), 95% confidence interval (CI) and AUC values at various cut-off values for PMN CD64 in different categories of infection.
Discussion
The diagnosis of neonatal sepsis is a challenge, as there is no single reliable test for its early confirmation or exclusion.[2,6] An early biomarker of bacterial infection with high diagnostic sensitivity and specificity would be a valuable tool for therapeutic decision making, enabling unnecessary use of antibiotics in patients clinically suspected to have infection to be avoided.[7,22,23] Currently blood culture is the most reliable method for detection of bacterial infections. However, the sensitivity of this method is low and using it as a gold standard in the diagnosis of bacteraemia is fraught with difficulties.[2,10] A study by Magudumana et al.[6] showed that fewer than 10% of neonates evaluated and treated for sepsis had blood culture-confirmed infection.
In this study we evaluated the diagnostic utility of PMN CD64 in neonatal sepsis. Of 76 participants suspected to have sepsis because of clinical signs, only 1 had a positive blood culture. An additional 5 patients had probable infection and further 30 had possible infection according to defined categories of infection. In neonatal sepsis a negative blood culture does not necessarily rule out infection. Using the patient group with both definite and probable infection as the gold standard-positive group, PMN CD64 at a cut-off of 1.8 showed specificity of 60.6%, sensitivity of 85.7%, a PPV of 31.6% and an NPV of 95.2%. This is the patient group that probably represents the true-positive group, and the high NPV of PMN CD64 in this group indicates that PMN CD64 should find a role in ruling out sepsis/infection when the PMN CD64 index is within the normal reference range. We believe that some of this group of babies had infection/sepsis despite negative blood cultures. As noted in the literature, there are multiple reasons for negative blood cultures, including possible effects of antibiotic therapy in utero.[1]
Our findings concur with the outcomes of numerous studies on the diagnostic performance of PMN CD64 in sepsis in view of the high sensitivity compared with blood culture and the high NPV.[13,15,16,19] Most of these studies, however, report high PPVs, which do not concur with our findings. The reason for the low PPV in our study could be the fact that ours was a prospective analysis that did not start off with patients who had proven infection. As a result, we had a lower prevalence of blood culture-confirmed infection compared with retrospective cohort analyses.
If the patients with possible infection are included with the definite and probable infection groups as the gold standard-positive group (1.8 cut-off), the sensitivity of PMN CD64 in the diagnosis of infection decreases without an improvement in specificity. The PPV improved from 32% to 58.1%, but the NPV dropped to 51%.This is not, however, the most likely representation of the true-positive patient group, as if all patients with 'possible' infection were really in need of antibiotics for microbial sepsis, there would be no need for laboratory diagnostic markers of infection, and clinical suspicion would suffice, a scenario not true of daily clinical practice.
While our study was conducted mainly in neonates with early-onset infection, a study by Ng et al. in neonates with late-onset infection showed that PMN CD64 had the highest sensitivity and specificity at the onset of suspected infection (95% and 88%, respectively) and at 24 hours (97% and 90%).[4] In the same study, PMN CD64 had the best overall performance compared with CRP, interleukin-6, CD11b, CD25 and CD45RO.
In our study there was no statistically significant correlation between PMN CD64 and other markers of infection (CRP, WCC and Plt). This suggests that CD64 is not supplying redundant information already given by CRP, WCC and Plt, but represents an independent variable with additional clinical impact.
Conclusions
We recommend the inclusion of PMN CD64 index into the diagnostic algorithm for neonatal sepsis. A negative PMN CD64 result had a high NPV in ruling out definite (100%) or probable and definite infection (95.2%) at a cut-off value of 1.8. Results of PMN CD64 are available in a much shorter time than blood culture results. As the PPV of the test was low in confirming infection, PMN CD64 should be used as a screening rather than a confirmatory test for infection. A cost-effective analysis would be of future benefit.
Author contributions. MBD devised protocol, collected data, performed the neutrophil CD64 test and wrote up the manuscript, TMW and MSS assisted with the protocol, co-supervised the project and reviewed the manuscript, DEB assisted with protocol, co-supervised the project and reviewed the manuscript, and OOF did data analysis and reviewed the manuscript.
Conflict of interest. The authors declare that there is no conflict of interest.
Funding. The study was funded by a National Health Laboratory Service trust fund and an individual research grant from the Faculty of Health Sciences, University of the Witwatersrand, for an MMed degree for M B Dhlamini.
Ethics approval. The study was approved by the Human Research Ethics Committee of the University of the Witwatersrand, Johannesburg, protocol number M081106.
References
1. Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates: Neutrophil CD64 as a diagnostic marker. Pediatrics 2008;121(1):129-134. [http://dx.doi.org/10.1542/peds.2007-1308] [ Links ]
2. Ng PC, Lam HS. Diagnostic markers for neonatal sepsis. Curr Opin Pediatr 2006;18(2):125-131. [http://dx.doi.org/10.1097/01.mop.0000193293.87022.4c] [ Links ]
3. Bomela HN, Ballot DE, Cory BJ, Cooper PA. Use of C-reactive protein to guide duration of empiric antibiotic therapy in suspected early neonatal sepsis. Pediatr Infect Dis J 2000;19(6):531-535. [http://dx.doi.org/10.1097/00006454-200006000-00008] [ Links ]
4. Ng PC, Li K, Wong RP, Chui KM, Wong E, Fok TF. Neutrophil CD64 expression: A sensitive diagnostic marker for late-onset nosocomial infection in very low birthweight infants. Pediatr Res 2002;51(3):296-303. [http://dx.doi.org/10.1203/00006450-200203000-00006] [ Links ]
5. Ballot DE, Perovic O, Galpin J, Cooper PA. Serum procalcitonin as an early marker of neonatal sepsis. S Afr Med J 2004;94(10):851-854. [ Links ]
6. Magudumana MO, Ballot DE, Cooper PA. Serial interleukin 6 measurements in the early diagnosis of neonatal sepsis. J Trop Pediatr 2000;46(5):267-271. [http://dx.doi.org/10.1093/tropej/46.5.267] [ Links ]
7. Layseca-Espinosa E, Perez-Gonzalez LF, Torres-Montes A, et al. Expression of CD64 as a potential marker of neonatal sepsis. Pediatr Allergy Immunol 2002;13(5):319-327. [http://dx.doi.org/10.1034/j.1399-3038.2002.01064.x] [ Links ]
8. Bakke AC, Allen E, Purtzer MZ, Dodhar BSA. Neutrophil CD64 expression: Distinguishing acute inflammatory autoimmune disease from systemic infections. Clinical and Applied Immunology Reviews 2001;1:267-275. [http://dx.doi.org/10.1016/S1529-1049(01)00029-0] [ Links ]
9. Hoffmann JJ. Neutrophil CD64: A diagnostic marker for infection and sepsis. Clin Chem Lab Med 2009;47(8):903-916. [http://dx.doi.org/10.1515/CCLM.2009.224] [ Links ]
10. Aikawa N, Fujishima S, Endo S, et al. Multicenter prospective study of procalcitonin as an indicator of sepsis. J Infect Chemother 2005;11(3):152-159. [http://dx.doi.org/10.1007/s10156-005-0388-9] [ Links ]
11. Jankovic B, Veljkovic D, Pasic S, Ralconjac Z, Jevtic D, Martic J. [C-reactive protein and cytokines in the diagnosis of neonatal sepsis]. Med Pregl 2006;59(11-12):545-549. [ Links ]
12. Nuntnarumit P, Pinkaew O, Kitiwanwanich S. Predictive values of serial C-reactive protein in neonatal sepsis. J Med Assoc Thai 2002;85(Suppl 4):S1151-1158. [ Links ]
13. Davis BH, Olsen SH, Ahmad E, Bigelow NC. Neutrophil CD64 is an improved indicator of infection or sepsis in emergency department patients. Arch Pathol Lab Med 2006;130(5):654-661. [ Links ]
14. Ng PC, Li G, Chui KM, et al. Neutrophil CD64 is a sensitive diagnostic marker for early-onset neonatal infection. Pediatr Res 2004;56(5):796-803. [http://dx.doi.org/10.1203/01.PDR.0000142586.47798.5E] [ Links ]
15. Livaditi O, Kotanidou A, Psarra A, et al. Neutrophil CD64 expression and serum IL-8: sensitive early markers of severity and outcome in sepsis. Cytokine 2006;36(5-6):283-290. [http://dx.doi.org/10.1016/j.cyto.2007.02.007] [ Links ]
16. Davis BH, Bigelow NC. Comparison of neutrophil CD64 expression, manual myeloid immaturity counts, and automated hematology analyzer flags as indicators of infection or sepsis. Lab Hematol 2005;11(2):137-147. [http://dx.doi.org/10.1532/LH96.04077] [ Links ]
17. Masuda M, Roos D. Association of all three types of Fc gamma R (CD64, CD32, and CD16) with a gamma-chain homodimer in cultured human monocytes. J Immunol 1993;151(12):7188-7195. [ Links ]
18. Danikas DD, Karakantza M, Theodorou GL, Sakellaropoulos GC, Gogos CA. Prognostic value of phagocytic activity of neutrophils and monocytes in sepsis. Correlation to CD64 and CD14 antigen expression. Clin Exp Immunol 2008;154(1):87-97. [http://dx.doi.org/10.1111/j.1365-2249.2008.03737.x] [ Links ]
19. Allen E, Bakke AC, Purtez MZ, Deodhar A. Neutrophil CD64 expression: Distinguishing acute inlammatory autoimmune disease from systemic infections. Ann Rheum Dis 2002;61(6):522-525. [http://dx.doi.org/10.1136/ard.61.6.522] [ Links ]
20. Cardelli P, Ferraironi M, Amodeo R, et al. Evaluation of neutrophil CD64 expression and procalcitonin as useful markers in early diagnosis of sepsis. Int J Immunopathol Pharmacol 2008;21(1):43-49. [ Links ]
21. Trillium Diagnostics. Leuko64 Assay for Detection of Inflammation and Tissue Injury. Package insert, 2009. [ Links ]
22. Cid J, Aguinaco R, Sanchez R, Garcia-Pardo G, Llorente A. Neutrophil CD64 expression as marker of bacterial infection: A systematic review and meta-analysis. J Infect 2010;60(5):313-319. [http://dx.doi.org/10.1016/jjinf.2010.02.013] [ Links ]
23. Chan T, Gu F. Early diagnosis of sepsis using serum biomarkers. Expert Rev Mol Diagn 2011;11(5):487-496. [http://dx.doi.org/10.1586/erm.11.26] [ Links ]
 Correspondence: MB Dhlamini (matshediso.dhlamini@nhls.ac.za)
Correspondence: MB Dhlamini (matshediso.dhlamini@nhls.ac.za)














