Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.7 n.1 Pretoria Jan. 2013
RESEARCH
Effect of prophylactic phenobarbital on seizures, encephalopathy and mortality in neonates with perinatal asphyxia
S VelaphiI; M MokhachaneII; R MphahleleIII; E Beckh-ArnoldIV
IMB ChB, FCPaed (SA); Department of Paediatrics, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg
IIMB BCh, FCPaed (SA); Department of Paediatrics, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg
IIIMB BCh, FCPaed (SA); Department of Paediatrics, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg
IVMB ChB, FCPaed (SA); Department of Human Genetics, National Laboratory Health Services, Johannesburg
ABSTRACT
BACKGROUND: Seizures after an asphyxial insult may result in brain damage in neonates. Prophylactic phenobarbital may reduce seizures.
OBJECTIVE: To determine the effect of prophylactic phenobarbital on seizures, death and neurological outcome at hospital discharge.
METHODS: Neonates with base deficit >16 mmol/l and Apgar score at 5 minutes <7 or requiring resuscitation for >5 minutes at the time of birth were randomised to prophylactic phenobarbital 40 mg/kg (n=50) or placebo (controls) (n=44) within the first 6 hours of life. They were monitored for clinical seizures, hypoxic ischaemic encephalopathy (HIE) and mortality.
RESULTS: Seizures developed in 30.0% of the phenobarbital group as opposed to 47.7% of the control group (relative risk 0.63; 95% confidence interval -0.37 - 1.06; p=0.083). The proportions of patients who had died and/or had HIE II or III at time of discharge from hospital were similar in the two groups (42.0% v. 45.5%). There were no differences in mortality between the two groups (14.0% v. 15.9%).
CONCLUSION: In infants with asphyxia, prophylactic phenobarbital does not reduce the incidence of seizures, HIE and mortality.
Perinatal asphyxia remains a common cause of neonatal death and is also one of the causes of brain damage resulting in long-term neurological deficits in children. The brain injury that develops due to asphyxia is related to increased cerebral blood flow, increased cerebral metabolism and re-oxygenation, which lead to cerebral oedema and excessive production of free radicals.[1-3] Newborn babies with brain injury secondary to intrapartum asphyxia may present with neonatal seizures as early as 4 hours after birth. The incidence of seizures following perinatal asphyxia has been reported to be as high as 50 - 68%.[4,5] Seizures may result in an increase in metabolic demands, leading to further neurological damage. Repeated seizures may interfere with brain growth and development, resulting in neurological impairment.[6,7] Neurological damage secondary to occurrence of seizures suggests that treating or preventing seizures may be beneficial in infants with perinatal asphyxia.
Seizures in neonates are often difficult to diagnose because their presentation is frequently subtle, and some of them can only be diagnosed electrographically. Electrographic seizures occurring in the absence of clinical seizures in neurologically abnormal newborns are reported to be common.[8-11] Electrographic seizures have been associated with high mortality and morbidity.[9,12-14] Treating these seizures could therefore reduce the mortality and morbidity associated with perinatal asphyxia. Seizures of this type can only be diagnosed through the use of an electro-encephalograph (EEG). In developing countries monitoring of patients with perinatal asphyxia/hypoxic encephalopathy using an EEG is often not possible because of lack of or inadequate resources. Giving an anticonvulsant empirically in all infants with perinatal asphyxia who are at risk of developing seizures could reduce the mortality and morbidity associated with these electrographic seizures.
Phenobarbital is an anticonvulsant commonly used in neonates with seizures. Prophylactic phenobarbital in newborns with perinatal asphyxia has been reported to reduce the incidence of seizures.[15,16] In addition to its anticonvulsant effects, it potentially has a neuroprotective efect through reducing the metabolic rate, inhibiting lipid peroxidation, stabilising cell membranes, and free-radical scavenging action.[17-19] This possible neuroprotective effect of phenobarbital has been supported by a study that reported a significant improvement in neurological outcome at 3 years of age.[20] There is also a concern that early administration of phenobarbital in infants with perinatal asphyxia may be associated with an increased incidence of seizures and mortality.[21] Studies that have reported a reduction of seizures and perinatal mortality with phenobarbital have been either retrospective studies or prospective randomised clinical trials with small sample sizes. In this study, we aimed to determine whether prophylactic single-dose intravenous phenobarbital given within 6 hours of birth to term and near-term neonates with perinatal asphyxia would decrease the death rate or abnormal indings on neurological examination at time of discharge.
Methods
The study was conducted at Chris Hani Baragwanath Academic Hospital, a public government hospital in Johannesburg, South Africa, - from March 2003 to August 2005. Infants with a gestational age of ≥ 34 weeks and/or weight ≥ 2000 g were eligible for the study if they had a base deficit of ≥ 16 mmol/l on measurement of arterial blood gas within an hour of delivery and an Apgar score of <7 at 5 minutes, or required resuscitation for more than 5 minutes. Infants who had congenital abnormalities, could not be randomised to intervention within 6 hours after birth or had no spontaneous respiration within 20 minutes after birth with or without bradycardia were excluded. The enrolled infants were randomly assigned to a treatment or placebo group using sealed serially numbered opaque envelopes that were opened only at the time of randomisation. The infants in the intervention group were given an infusion of phenobarbital 40 mg/kg over a period of an hour, starting within the first 6 hours after birth. The phenobarbital was prepared at a strength of 40 mg/ml. Infants in the placebo group were given 1 ml/kg of normal saline (sodium chloride solution), also infused over a period of an hour within the first 6 hours after birth. The rest of the management of the two study groups was the same. Infants were monitored for oxygen saturation, blood pressure, heart rate and respiratory rate every 15 minutes during administration of the study drug or the placebo, then every hour for 6 hours, then every 3 hours for 24 hours. All patients received nil per mouth for the first 24 hours of life, were started on a 10% dextrose-based solution at 60 ml/kg/24 h, had 3-hourly haemoglucotest monitoring, and had their serum electrolytes and creatinine measured 24 - 48 hours after birth. All patients were started on ampicillin and gentamicin, since sepsis was considered to be a possible cause of asphyxia; these were stopped at 48 - 72 hours if laboratory markers for sepsis (complete blood count, C-reactive protein and blood culture) were negative. Induced hypothermia was not used in any of the patients since it was not part of standard treatment. Patients who developed seizures had their serum electrolytes and glucose, calcium and magnesium levels measured. Infants received a neurological examination before the study drug was administered, but findings from this examination were not used as criteria for inclusion or exclusion. All the nurses and doctors looking after the patients were blinded to the group allocation. Infants were observed for development of clinical seizures by both nurses and doctors, and those who developed seizures were managed with phenytoin and clonazepam. If they continued to have seizures despite infusion of phenytoin 20 mg/kg and a dose of intravenous clonazepam 0.1 mg/kg, the code was broken and those who were randomised to saline were managed with phenobarbital. In the infants who were on phenobarbital, blood was taken for measurement of the phenobarbital level and they were treated with phenobarbital if the level was below the therapeutic range. Neonatal consultants who were not involved in taking care of the infants were asked to do neurological examinations according to Sarnat staging in all patients 12 - 24 hours, 2 - 3 days and 5 - 7 days after birth and on discharge.
Statistical analysis
Description of the characteristics of the patients enrolled was performed using means, standard deviations (SDs) for parametric measures, and medians and percentiles for non-parametric measures. Comparisons for categorical variables were performed using a chi-square test, Student's t-test was used for continuous variables, and differences were considered to be significant if the p-value was <0.05.
Results
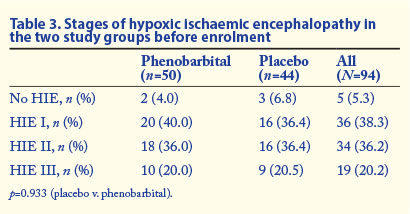
There were 94 infants who were randomly assigned, 50 to the phenobarbital group and 44 to the normal saline group (placebo). There were no significant differences between the two groups with regard to signs suggestive of fetal distress, namely meconium staining of the amniotic fluid and cardiotocographic abnormalities (Table 1). There were also no significant differences in mean birth weight, gestation, 5-minute Apgar score, extent of resuscitation required, pH or base deficit (Table 2). The mean age at administration of phenobarbital was 245 (SD 72) minutes, which was greater than the mean age at which normal saline was given (210 (SD 81) minutes) (p=0.043). The severity of hypoxic ischaemic encephalopathy according to Sarnat staging at the time of enrolment was similar between the two groups (Table 3). There were no significant changes in heart rate, respiratory rate or blood pressure during and after phenobarbital infusion (data not shown).
Of the 21 patients in the placebo group who had seizures, 6 continued to have seizures despite administration of phenytoin and clonazepam and were subsequently treated with phenobarbital after the code was broken. These patients were analysed in the control group as the analysis was done on an intention-to-treat basis. In the phenobarbital group, 2 of the 15 patients who had seizures were not controlled on phenytoin and clonazepam and were treated with phenobarbital after phenobarbital serum levels were measured and found to be low. Outcomes (morbidity and mortality) to hospital discharge in the two groups are compared in Tables 4 and 5. Of the patients who were given phenobarbital, 30.0% developed seizures, compared with 47.7% in the control group (relative risk (RR) 0.63; 95% confidence interval (CI) 0.37 - 1.06; p=0.083). None of the patients who had seizures had hypoglycaemia, hypocalcaemia or electrolyte abnormalities, and overall there were no diferences in serum electrolytes and glucose levels between the two groups.
The proportions of patients who had died or were assessed as having hypoxic ischaemic encephalopathy (HIE) II or III at discharge were similar between the two groups, 42.0% in the phenobarbital group compared with 45.5% in the control group (RR 0.92; 95% CI 0.58 - 1.46; p=0.736). There was no statistically significant difference between the two groups in the proportions of patients who had HIE I at enrolment and had progressed to HIE II or III by the time of discharge (RR 0.69; 95% CI 0.29 - 1.66; p=0.413).
Of the 94 patients enrolled, 14 (14.9%) died, all of them due to severe HIE. There were no differences in mortality rates between the two groups (14.0% v. 15.9%). All the patients who died were assessed as having severe encephalopathy (HIE III) before enrolment except for one, who was assessed as having HIE I but had progressed to HIE III by the time she died; this patient was in the phenobarbital group. Among the patients with HIE II or III, there were no statistically significant diferences between the two groups in improvement to normal or HIE I (39.3% v. 28.0%; RR 1.40; 95% CI 0.64 - 3.06; p=0.394). Mortality rates in patients with HIE II or III were not significantly different between the two groups (21.4% v. 28.0%; RR 0.76; 95% CI 0.30 - 1.97; p=0.580) (Table 5).
Discussion
Although hypothermia is becoming part of standard care in the management of full-term infants with moderate to severe hypoxic ischaemic encephalopathy, it is not readily available in developing countries, where the need is greater than in developed countries because of the high incidence of asphyxia.[22-24] Efforts to find less expensive therapies to reduce brain injury secondary to asphyxia should therefore continue.
One of the contributors to brain injury is seizures. Electrographic seizures have been reported to occur in 65% of patients with HIE treated with hypothermia, and 47% of these seizures were non-convulsive.[25] Prophylactic phenobarbital with or without hypothermia may therefore reduce brain injury through its anticonvulsant effects. In this study we enrolled 94 patients to evaluate the effect of prophylactic phenobarbital on short-term neurological outcomes, i.e. development of seizures, severity of hypoxic encephalopathy at the time of hospital discharge, and mortality, in infants with perinatal asphyxia. We found that prophylactic phenobarbital given within 6 hours of birth in infants with perinatal asphyxia did not affect these outcomes.
A number of studies looking at the effect of prophylactic phenobarbital or other barbiturates in reducing the development - of seizures in infants with perinatal asphyxia have reported contradictory results.[15, 16, 20, 21, 26] In 1982, Svenningsen et al. reported that administration of phenobarbital within 60 minutes of delivery in infants with severe neonatal asphyxia, without awaiting clinical signs of convulsions, was associated with a 67% reduction in recurrent neonatal seizures.[15] This was a retrospective study, in contrast to our study, which was a prospective study. A relatively recent study by Singh et al. also reported that phenobarbital given within 6 hours of birth to term and near-term neonates with HIE - reduced the incidence of seizures by 80%, but they only enrolled 45 infants, a smaller number than in our study.[16] In a retrospective chart review of term infants with perinatal asphyxia, Ajayi et al. reported the contrasting finding that phenobarbital given within 1 hour after resuscitation with the aim of preventing HIE was associated with a threefold increase in the incidence of subsequent seizures, and that seizures per se were associated with almost 20-fold increase in mortality.[21] Studies by Hall et al. and Goldberg et al. reported similar results to ours, prophylactic phenobarbital or thiopental not being associated with reductions in the incidence of seizures.[20,26] The reasons for the differences in rates of occurrence of seizures between the studies are unclear, but they could be due to the study design or numbers enrolled. The numbers enrolled in the studies by Svenningsen et al. and Singh et al. are small, and the study by Ajayi et al. was a retrospective review, and the decision to give phenobarbital was based on the choice of the attending doctor; doctors could therefore have given phenobarbital to infants who had severe asphyxia and were therefore more likely to have seizures or to die. Recently, Meyn et al. reported that prophylactic phenobarbital reduced clinical seizures in infants with HIE managed with induced hypothermia, although it did not have an effect on death and neurological impairment.[27]
We did not find that use of prophylactic phenobarbital was associated with a difference in mortality and HIE or its severity. Our findings are similar to those reported by Hall et al.[20] and Singh et al.[16] Svenningsen et al.[l5] reported a reduction in mortality, while Ajayi et al.[21] reported an increase in mortality. The most likely reason for this difference was that the Ajayi study was done in a country with limited resources, so monitoring and other aspects of the management of the asphyxiated infants may have been inadequate; for example, they were not given mechanical ventilation, and their patients had more severe asphyxia than those in our study. Use of prophylactic phenobarbital in the absence of inadequate monitoring or mechanical ventilation may be associated with high mortality, and it may not have an impact in infants who have had severe asphyxia. Sarkar et al reported indings similar to ours, i.e. that phenobarbital did not improve outcome in terms of neonatal death in infants with HIE.[28]
Hall et al. reported that prophylactic phenobarbital was associated with a 67% reduction in abnormal neurological outcomes,[20] results similar to those reported by Svenningsen et al.[15] Two studies that have reported on use of phenobarbital in infants with HIE treated with induced hypothermia reported that it had no effect on the composite outcome of death and neurological impairment.[27,28] We were unable to report on long-term neurological outcomes because of a high attrition rate. The effect of phenobarbital on long-term outcome needs to be studied further, especially in developing countries where resources are limited. When conducting studies in developing countries it is necessary to make adequate resources available to provide interventions to be studied and to ensure that patients come back for follow-up in order to complete the studies as planned and avoid high attrition rates.
The major limitation of our study was that seizures were only monitored clinically, so electrographic seizures with no clinical manifestation could have been missed. Future studies on the use of prophylactic phenobarbital should monitor for seizures electrographically.
On the basis of our findings, we conclude that prophylactic phenobarbital 40 mg/kg in infants with asphyxia has no effect on the incidence of seizures, the incidence and severity of HIE at the time of hospital discharge, or mortality. If prophylactic phenobarbital does have an effect in improving long-term neurodevelopmental outcome, it is probably not through its anticonvulsant effect but through other mechanisms, namely inhibiting lipid peroxidation, stabilising cell membranes, and free-radical scavenging.[16,17]
Acknowledgement. We would like to thank the Medical Research Council of South Africa for assisting us with a research grant to conduct this study.
References
1. Fellman V, Raivio KO. Reperfusion injury as the mechanism of brain damage after perinatal asphyxia. Pediatr Res 1997;41(5):599-606. [http://dx.doi.org/10.1203/00006450-199705000-00001] [ Links ]
2. Roth SC, Edwards AD, Cady EB, et al. Relation between cerebral oxidative metabolism following birth asphyxia, and neurodevelopmental outcome and brain growth at one year. Dev Med Child Neurol 1992;34(4):285-295. [http://dx.doi.org/10.1111/j.1469-8749.1992.tb11432.x] [ Links ]
3. Grow J, Barks JD. Pathogenesis of hypoxic-ischemic cerebral injury in the term infant: Current concepts. Clin Perinatol 2002;29(4):585-602. [http://dx.doi.org/10.1016/S0095-5108(02)00059-3] [ Links ]
4. Brown JK, Purvis RJ, Forfar JO, Cockburn F. Neurological aspects of perinatal asphyxia. Dev Med Child Neurol 1974;16(5):567-580. [http://dx.doi.org/10.1111/Fj.1469-8749.1974.tb04176.x] [ Links ]
5. Finer NN, Robertson CM, Richards RT, Pinnell LE, Peters KL. Hypoxic-ischemic encephalopathy in term neonates: Perinatal factors and outcome. J Pediatr 1981;98(1):112-117. [http://dx.doi.org/10.1016/S0022-3476(81)80555-0] [ Links ]
6. Holmes GL, Gairsa JL, Chevassus-Au-Louis N, Ben-Ari Y. Consequences of neonatal seizures in the rat: Morphological and behavioral effects. Ann Neurol 1998;44(6):845-857. [http://dx.doi.org/10.1002/ana.410440602] [ Links ]
7. Dzhala V, Ben-Ari Y, Khazipov R. Seizures accelerate anoxia-induced neuronal death in the neonatal rat hippocampus. Ann Neurol 2000;48(4):632-640. [http://dx.doi.org/10.1002/1531-8249(200010)48:4<632::AID-ANA10>3.0.CO;2-3] [ Links ]
8. Hellstrom-Westas L, Rosen I, Swenningsen NW. Silent seizures in sick infants in early life. Diagnosis by continuous cerebral function monitoring. Acta Paediatr Scand 1985;74(5):741-748. [http://dx.doi.org/10.1111/j.1651-2227.1985.tb10024.x] [ Links ]
9. Connell J, Oozeer R, de VL, Dubowitz LM, Dubowitz V. Continuous EEG monitoring of neonatal seizures: Diagnostic and prognostic considerations. Arch Dis Child 1989;64(4 Spec. No):452-458. [http://dx.doi.org/10.1136/adc.64.4_Spec_No.452] [ Links ]
10. Weiner SP, Painter MJ, Geva D, Guthrie RD, Scher MS. Neonatal seizures: Electroclinical dissociation. Pediatr Neurol 1991;7(5):363-368. [http://dx.doi. org/10.1016/0887-8994(91)90067-U] [ Links ]
11. Bye A, Flanagan D. Electroencephalograms, clinical observations and the monitoring of neonatal seizures. J Paediatr Child Health 1995;31(6):503-507. [http://dx.doi.org/10.1111/j.1440-1754.1995.tb00872.x] [ Links ]
12. Legido A, Clancy RR, Berman PH. Neurologic outcome after electroencephalographically proven neonatal seizures. Pediatrics 1991;88(3):583-596. [ Links ]
13. Scher MS, Aso K, Beggarly ME, Hamid MY, Steppe DA, Painter MJ. Electrographic seizures in preterm and full-term neonates: Clinical correlates, associated brain lesions, and risk for neurologic sequelae. Pediatrics 1993;91(1):128-134. [ Links ]
14. McBride MC, Laroia N, Guillet R. Electrographic seizures in neonates correlate with poor neurodevelopmental outcome. Neurology 2000;55(4):506-513. [http://dx.doi.org/10.1212/WNL.55.4.506] [ Links ]
15. Svenningsen NW, Blennow G, Lindroth M, Gaddlin PO, Ahlstrom H. Brain-orientated intensive care treatment in severe neonatal asphyxia. Effects of phenobarbitone protection. Arch Dis Child 1982;57(3):176-183. [http://dx.doi.org/10.1136/adc.57.3.176]
16. Singh D, Kumar P, Narang A. A randomized controlled trial of phenobarbital in neonates with hypoxic ischemic encephalopathy. J Matern Fetal Neonatal Med 2005;18(6):391-395. [http://dx.doi.org/10.1080/13895260500327979] [ Links ]
17. Vannucci RC, Perlman JM. Interventions for perinatal hypoxic-ischemic encephalopathy. Pediatrics 1997;100(6):1004-1014. [http://dx.doi. org/10.1542/peds.100.6.1004] [ Links ]
18. Singh D, Kumar P, Majumdar S, Narang A. Effect of phenobarbital on free radicals in neonates with hypoxic ischemic encephalopathy a randomized controlled trial. J Perinat Med 2004;32(3):278-281. [ Links ]
19. Gathwala G, Marwah A, Gahlaut V, Marwah P. Effect of high-dose phenobarbital on oxidative stress in perinatal asphyxia: An open label randomized controlled trial. Indian Pediatr 2011;48(8):613-617. [http://dx.doi.org/10.1007/s13312-011-0106-x] [ Links ]
20. Hall RT, Hall FK, Daily DK. High-dose phenobarbital therapy in term newborn infants with severe perinatal asphyxia: A randomized, prospective study with three-year follow-up. J Pediatr 1998;132(2):345-348. [http://dx.doi.org/10.1016/S0022-3476(98)70458-5] [ Links ]
21. Ajayi OA, Oyaniyi OT, Chike-Obi UD. Adverse effects of early phenobarbital administration in term newborns with perinatal asphyxia. Trop Med Int Health 1998;3(7):592-595. [http://dx.doi.org/10.1046/j.1365-3156.1998.00274.x] [ Links ]
22. Azzopardi D, Strohm B, Edwards AD, et al. Treatment of asphyxiated newborns with moderate hypothermia in routine clinical practice: How cooling is managed in the UK outside a clinical trial. Arch Dis Child Fetal Neonatal Ed 2009;94(4):F260-F264. [http://dx.doi.org/10.1136/adc.2008.146977] [ Links ]
23. Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: Synthesis and meta-analysis of trial data. BMJ 2010;340:c363. [http://dx.doi.org/10.1136/bmj.c363] [ Links ]
24. Shah PS. Hypothermia: A systematic review and meta-analysis of clinical trials. Semin Fetal Neonatal Med 2010;15(5):238-246. [http://dx.doi.org/10.1016/j.siny.2010.02.003] [ Links ]
25. Wusthof CJ, Dlugos DJ, Gutierrez-Colina A, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol 2011;26(6):724-728. [http://dx.doi.org/10.1177/0883073810390036] [ Links ]
26. Goldberg RN, Moscoso P, Bauer CR, et al. Use of barbiturate therapy in severe perinatal asphyxia: A randomized controlled trial. J Pediatr 1986;109(5):851-856. [http://dx.doi.org/10.1016/S0022-3476(86)80713-2] [ Links ]
27. Meyn DF, Jr., Ness J, Ambalavanan N, Carlo WA. Prophylactic phenobarbital and whole-body cooling for neonatal hypoxic-ischemic encephalopathy. J Pediatr 2010;157(2):334-336. [http://dx.doi.org/10.1016/j.jpeds.2010.04.005] [ Links ]
28. Sarkar S, Barks JD, Bapuraj JR, et al. Does phenobarbital improve the effectiveness of therapeutic hypothermia in infants with hypoxic-ischemic encephalopathy? J Perinatol 2012;32(1):15-20. [http://dx.doi.org/10.1038/jp.2011.41] [ Links ]
 Correspondence: S Velaphi (Sithembiso.Velaphi@wits.ac.za)
Correspondence: S Velaphi (Sithembiso.Velaphi@wits.ac.za)














