Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Bioethics and Law
On-line version ISSN 1999-7639
SAJBL vol.15 n.2 Cape Town Oct. 2022
http://dx.doi.org/10.7196/SAJBL.2022.v15i2.802
ARTICLE
Informed consent in clinical trials
G P KovaneI; V C NikodemII; O KhondoweIII
IPhD; NuMIQ Focus Area, School of Nursing Science, North-West University, South Africa; Stellenbosch University, Cape Town, South Africa
IIDCur, LLB; Nelson Mandela School of Law, Faculty of Law, University of Fort Hare, South Africa
IIIPhD; Faculty of Health Sciences, Lusaka Apex Medical University, Zambia
ABSTRACT
BACKGROUND. Informed consent (IC) is not only a regulatory but also an ethical requirement to participate in any clinical trial. It is essential to determine that research participants understand what they consent to. Studies that evaluate participants' understanding of IC conclude that recall and understanding of IC is often low, and researchers recommend that interactive multimedia interventions should be implemented to optimise understanding.
OBJECTIVES. To assess participants' understanding of IC of the research trial that they agreed to participate in.
METHODS. A descriptive survey design, within a quantitative research approach, was used to conduct the study at two government hospitals in the Eastern Cape Province. A semi-structured, self-administered questionnaire was used to collect information from 170 participants in research studies. Descriptive statistics were used to analyse the results.
RESULTS. Participants were recruited from among women who enrolled in any of the three studies that were ongoing at the two sites during the recruitment period. The study participants had a mean age of 25.9 years. Nearly one-third (30%) could not recall the purpose of the original trial that they consented to. The concept of randomisation was not understood by any of the participants.
CONCLUSION. Regardless of extensive efforts to ensure that participants understood their participation, this study unveiled poor recall of essential information on IC. It is proposed that IC should be short and only address essential components such as purpose, procedure, possible risks or benefits, alternative options if not participating and explaining the concept of voluntary participation.
Clinical research is a vital component to advance healthcare, and the execution of a successful clinical trial depends on recruiting and retaining research participants ethically and voluntarily.[1] Research participants are required to receive enough information about the trial, including what is expected from them, to enable them to give voluntary consent to participate. Apart from the regulatory, ethical and moral aspects of obtaining informed consent (IC), the question remains whether effective communication will be seen as the start of a trust relationship between the researcher and the participant that needs to continue throughout the research study.
The concept of consent can be traced back to 1914, when Justice B Cardozo[2] wrote that in the court's opinion 'Every human being of adult years and sound mind has a right to determine what shall be done with his own body, and a surgeon who performs an operation without his patient's consent commits an assault for which he is liable in damages.' The Nuremberg Doctors' Trials[3] referred to the idea that IC must be done voluntarily, be informed and be comprehended by human participants required to partake in research trials. The Californian Salgo trial[4] coined the term 'informed consent' when the court concluded that 'A physician violates his duty to his patient and subjects himself to liability if he withholds any facts which are necessary to form the basis of an intelligent consent by the patient to the proposed treatment. Likewise the physician may not minimise the known dangers of a procedure or operation in order to induce his patient's consent.'
Obtaining IC to ensure patient autonomy and self-determination was brought to light in South Africa (SA) as late as 1976 in the case of Richter and another v Estate Hammann.[5] It took another 17 years before the court secured the doctrine of IC in SA medical and health law jurisprudence, and stated that participants have fundamental rights to be informed about benefits and possible risks of any procedure.[6] Moreover, the National Health Act No. 61 of 2003[7] provides for research or experimentation on human subjects, while the doctrine of IC is codified in the Act in sections 6, 7, 8 and 71. The Act is in line with the right to self-determination that protects every person's right to bodily and psychological integrity as enshrined in chapter 2 of the SA Constitution.[8]
IC is the keystone of ethical clinical research and refers to a process of information exchange that requires dialogue between the participant and the researcher and involves efforts to promote understanding of participation in randomised controlled clinical trials (RCTs). IC safeguards patients' legal rights and promotes their freedom to decide to participate and is vested in ethical principles of respect for people, beneficence and justice.[91 However, Neff[10] affirms that consent is more than merely signing a form.
Adequate time should be given to the participant to read and to ask questions during the process of obtaining IC. Moreover, the process of obtaining IC includes non-coercive communication between the participant and the investigator, and IC must be presented in a simple format, including all the concepts as required by the World Medical Association (WMA) Declaration of Helsinki - Ethical Principles for Medical Research Involving Human Subjects.[11]
Attaining IC can result in misunderstandings by participants, and ambiguity, inconsistency and exaggeration may lead to confusion about what participants understand when consenting to participate in RCTs. Research has shown that vulnerable participants in poor socioeconomic communities may consent to RCTs as they may think they will have free access to medical care offered during the period they participate in the trials.[12] Moreover, Appelbaum et al.[13] believe that research participants tend to confuse research for routine clinical treatment, as they do not fully understand the concept of scientific methodologies such as randomisation. Furthermore, Wilkens and Forester [14] report that participants tend to overestimate the possibility of benefit and underrate probable associated risks. Participants also expected better care and believed that the new treatment would be better than the existing treatment."[15]
The present study was conducted to identify any existing gaps during communication of IC in women participating in clinical trials, with the hope to assist researchers in the future to explore new concepts that can be incorporated when obtaining IC.
The project was approved by the Stellenbosch University Health Research Ethics Committee (ref. no. N10/02/025), and permission to carry out the study was also obtained from the government hospitals in the Eastern Cape Province where data were collected.
Methodology
The present study aimed to achieve an understanding of participants' knowledge concerning IC when they participate in research studies. The study was carried out at two public state hospitals serving low-income populations in the Eastern Cape Province of SA. A convenient sampling method was used to recruit participants. Participants were recruited from women who had been enrolled in any of the three studies that were ongoing at the time of recruitment. The first study was an RCT comparing the efficacy, safety and acceptance of an intrauterine contraceptive device v. injectable depo progestogen in reducing pregnancy. The second RCT was to assess whether massaging the uterus may be as effective as oxytocin injection to prevent postpartum haemorrhage. The third trial was a descriptive study to determine the demographic and socioeconomic factors, infant feeding practices, immunisation and micronutrient status on infant growth, diarrhoea and respiratory diseases in infants aged 6 weeks to 9 months.
A descriptive survey design, within a quantitative research approach, was used to conduct the study at two public hospitals in the Eastern Cape Province. Participants were informed about the IC study via notifications in strategic places in the hospital. All women who participated in any one of the three ongoing original studies were invited to participate in the IC study. Participants were approached by a research midwife (GPK) between 8 and 48 hours after being enrolled in one of the original studies, to ensure that recall was still optimal. A copy of the IC was given to the participant to read, and thereafter the researcher explained the current IC to the participant. Sufficient time was given to the participants to read or ask questions regarding their participation in the current IC study.
The inclusion criteria for this study were women who consented to partake in any one of three ongoing studies at the two public hospitals and who were able to converse in either isiXhosa or English. The IC and questionnaire were available in both languages. If participants met the inclusion criteria, they signed two copies of the IC, one for them to keep and one for the researcher to file separately from the data collection sheet. The research midwife then recorded each participant's contact details onto the enrolment sheet and handed a semi-structured questionnaire to her to complete.
Participants were allowed to ask questions for clarification purposes when they were not sure of the content requested in the questionnaire. All the research midwives and the researcher (GPK) had completed a certificate in good clinical practice and undergone training to assist in executing clinical trials. Confidentiality was maintained at all times, and the information received from the participants was only made available to the research team, including the statistician. Participants were reminded that they were free to stop participation at any time, and were given assurance that it would not affect the service they were entitled to receive at the institution.
A semi-structured, self-administered questionnaire was used to collect information. The questionnaire consisted of open- and closed-ended questions and collected baseline socioeconomic and demographic information, as well as information based on the requirements as depicted in the World Health Organization (WHO)'s template for IC for participating in clinical trials. The quantitative data were captured onto an Excel sheet (Microsoft, USA) and analysed using SPSS version 14.0 (SPSS, USA). Descriptive analysis was used to analyse nominal and ordinal data. Information from open-ended questions was analysed through thematic analysis and then quantified.
Results
A total of 170 participants were recruited and agreed to be enrolled in the study. A total of 70 participants were in the original RCT comparing efficacy, safety and acceptance of the intrauterine contraceptive device and injectable depo progestogen in reducing pregnancy rate, 80 took part in the original RCT assessing whether massaging the uterus for 30 minutes may be as effective as oxytocin injection to prevent postpartum haemorrhage, and 20 participants were recruited from the original survey study related to infant feeding.
Bio- and socioeconomic information demonstrated that the study participants' mean age was 25.9 (standard deviation 5.98) years, and the range between 17 and 42 years. Slightly more than two-thirds (68%) had completed secondary education, and similarly, 65% reported that they had not been employed before they fell pregnant. Due to the nature of the original trials, one could have expected that 44% reported being in pain or discomfort when IC was obtained (Table 1).[16]
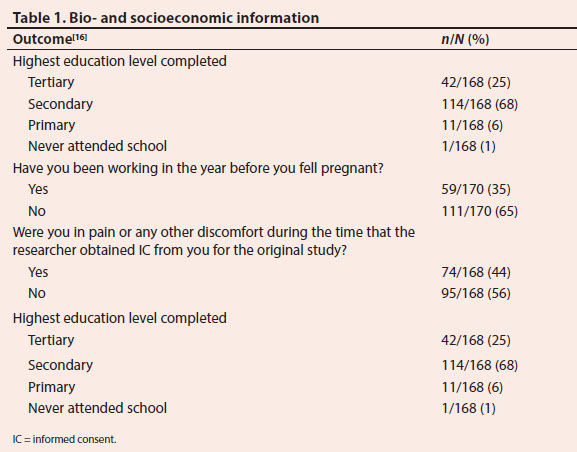
It is a protocol requirement to give a copy of the signed IC to the participant to keep, but the results showed that only 79% of participants confirmed that they were handed a copy of the IC of the original study. Sadly, less than half (49%) confirmed that they were given the opportunity to deliberate on their participation in the original study with their partner or confidant. Participants appeared not to understand why they had agreed to participate in a research project, as less than half (41%) could recall a reason as to why they consented to participate in the original trial. It is disturbing that 40% reported that they participated in order to receive treatment. Participants were not able to communicate the purpose of the original studies. Moreover, most participants did not recall what their responsibilities were while participating in the original trials (Table 1).[16]
Slightly more than half (55%) of the participants responded that researchers explained to them about the different available treatments. Of these, 74% could recall the correct treatment options. It appears that participants did not understand the concept of randomisation. None of the participants could explain what they understood about randomisation, or what the word meant. The majority of participants denied being informed of possible benefits of participating (97%); moreover, only a few participants agreed that they were informed about any risks related to the original study (22%). Most (75%) of the participants could not mention any risks that they were informed about, and participants stated that no alternatives were mentioned if they declined to participate in the original study (53%) (Table 1).[16]
Very few participants recalled that they had the right to withdraw (20%), and few (12%) stated that they understood what help would be available if there were trial-related injuries that occurred unexpectedly to them. Less than half (44%) recalled that the researchers and medical team would have access to the information that was collected from them in the original study, but more than half (55%) had no idea how the information would be used in the future. The majority (82%) of the participants agreed that they were given details of whom to contact if they had any additional questions in the original study, but some (29%) stated that they did not understand why they signed the IC. Most (88%) of the participants believed that the information that was given to them before they enrolled in the original study was adequate for them to make an informed decision regarding their trial participation (Table 2).[16]
Discussion
The four main ethical principles of obtaining IC are those of beneficence, non-maleficence, autonomy and justice.[17] The subjective patient-centred test was affirmed in Broude v McIntosh.[18] The National Health Act addresses the need for IC when research is done on human subjects and sets out the nature and scope of the information that should be disclosed to the participant. The Act emphasises that IC should be done in a language understood by the participant, and that the participant's level of literacy should always be considered. The challenge with most IC is whether adequate information has been disclosed to the participant, and whether the participant has understood the information before deciding on research participation.
The National Health Act mandates active consent from a parent or legal guardian for all research conducted on research participants <18 years old. This age restriction may cause some conflict when researchers wish to explore sexual and reproductive health research in SA, such as contraceptive use, teenage pregnancy and sexually transmitted diseases. This is because girl children <18 may obtain contraceptives without consent from their parents, but cannot participate in related research as they do not wish their parents to know about their use of contraceptives. This may interfere with girl children's rights to self-determination.
The original studies involved women who were in labour, had children, or who wanted to use contraceptives. It was noted that one participant was only 17 years old, so strictly she was not allowed to participate in the original research study, and rightly the researchers acknowledged that the research protocol was breached. The mean age of participants in this study was slightly younger than the mean age of childbearing women in SA, which is currently 27 years, but it is in line with the decrease in age due to the increase of teenage pregnancies reported in the country.[19]
Level of education has been linked with the comprehension and understanding of IC.[20] Furthermore, it has been reported that participants with low literacy are at risk of having poor comprehension of the IC concept.[21] Literacy and low education levels probably did not have an effect on this study, however, as the majority of participants had completed secondary or tertiary education and therefore should have had good literacy comprehension.
Britz and Le Roux-Kemp[22] emphasised that pain, psychological factors or socioeconomic factors may influence the process of getting IC from a participant. The decision-making capacity of women in labour or during the postpartum period may thus be compromised: stress, pain and anxiety may influence the decision-making process. In this study, 44% of the women stated that they were experiencing pain or discomfort at the time that they were asked to participate in the original studies. As patients were recruited during a vulnerable time, such as during labour, the question is whether all participants were sensitised during the antepartum period through pamphlets and posters that they might be requested to participate in research trials.
It is acknowledged that participants may enrol in studies expecting special care, regardless of what they have been informed of, owing to socioeconomic vulnerability. The unemployment rate in the original studies is in line with unemployment rates in the Eastern Cape.[23] No funding was offered to participate in any of the trials, so it can be assumed that unemployment or the idea of incentives could not have served as a motivation to participate in the original studies. In SA, perinatal care is free for all women of low income at public facilities.
In many communities, participatory decision-making is part of culture, and it is becoming increasingly difficult to ignore the ethical and cultural challenges associated with this when applying the universal ethical guidelines, which are principled on individualistic autonomy and not cultural participatory research. The Declaration of Helsinki[11] allows the participant the opportunity to check in with their family members or community leaders prior to participation in research. It appears that participants' right to consult family members was not sufficiently valued in the original trials, as only 49% could recall that they were allowed to consult with family members. Community consultation and community consent are still ill-defined concepts for researchers, who need to be made aware of the difference between community awareness consultation and community consent.
Participants should always be informed about alternative treatments available to them when they decide whether or not to participate in a trial. The original trials examined in this study demonstrated that participants could mention the different treatments available to them, but Alexa-Stratulat et a/.[24] warn that despite participants being able to recall different treatment options, they may not understand that the treatment offered is related to research. The Belmont Report refers to research-based protective applications that include the disclosure of potential risks and benefits.[25] Participants cannot make an informed decision without all the required information. It is important that only those risks and benefits that may result or be directly linked to the trial be disclosed. A study by Gota et al.[15] reported that 83% of participants in their study were aware of potential risks and benefits, while only 22% of participants in this IC study could recall that they were informed about the risks and potential benefits of the original studies.
The SAGCP requires the researcher to attempt to ascertain why a research participant has withdawn prematurely from a trial. This question needs to be carefully stated, as the participant may feel threatened by the researcher attempting to obtain a reason for the withdrawal, and may for this reason decide not to withdraw. This requirement may indeed be a contradiction, as it does not fully respect the participant's wishes and voluntary decision to withdraw from a clinical trial prematurely. Allowing a participant to decline consent or to withdraw from research participation without a reason demonstrates respect for their rights. It appears that the SAGCP contradicts the notion of voluntary participation by requesting reasons why participants do not wish to participate or to withdraw early. SAGCP section 5.9.16 requires three signed copies: one for the participant, one for the patient hospital file and one for the research file. However, participants who take part in sensitive trials often do not wish to take home a signed IC form, as they do not wish others to know about their illness, so three copies are not always feasible.
Randomisation is extensively used in human clinical trials as a technique of experimental control to avoid selection bias and eliminate bias in assigning of treatment. Wendler[26] asserts that many research participants do not understand randomisation, but do understand that RCTs involve research, which is an important concept when obtaining IC, and concludes that participants do not need to fully understand randomisation to give valid IC.
Recommendations
We recommend that IC forms should be short and address only the essential components, such as the fact that the study involves research, the risks involved, any potential benefits, study procedures and alternatives to participation, and voluntary participation.
We agree with other researchers that an approach with more interactive features should be implemented to increase the chances of understanding IC.
We recommend that researchers take note of elements noted by Appiah,[27] such as that participants come from different backgrounds, different cultures, diverse socioeconomic statuses and varied individual decision-making capacities, that researchers should take into account beneficence and confidentiality, and that there may be a need for family involvement when signing IC documents.
Conclusion
Voluntary IC for participation in clinical research is the basis of health research ethics, and a requirement for clinical research in SA. The SA-specific guidance documents regarding voluntary IC requirements differ, and at times contradict international documents applicable in SA. Given the provisions of the SA Constitution[8] and applicable legislation, it is evident that voluntary IC provisions in the guidance documents are not always aligned with the legislation and Constitutional principles concerning IC.
Declaration. None.
Acknowledgements. The authors would like to thank all study participants. We are grateful for the contribution received from Goran and Daniel during data collection, and the Effective Care Research unit team and support.
Author contributions. GPK executed the research and wrote the manuscript, VCN conceptualised the idea of the research study and contributed towards the writing of the article and OK supervised GPK.
Funding. National Research Foundation and Norwegian Council of Universities' Programme for Development Research and Education (NUFU).
Conflicts of interest. None
References
1. Colombo C, Mayrhofer MT, Kubiak C, et al. The CORBEL matrix on informed consent in clinical studies: A multidisciplinary approach of research infrastructures building enduring life-science services. BMC Med Ethics 2021;22:95. https://doi.org/10.1186/s12910-021-00639-x
2. Schloendorff ME v The society of New York Hospital 1914 105 N.E. 92, 211 N.Y. 125.
3. United States of America v Brandt K et al (Case I) November 21, 1946-August 20, 1947.
4. Salgo v Leland Standford Junior University Board of Trustees 1957 154 Cal. App. 2d. 560, 317 P. 2d 170.
5. Richter and another v Estate Hammann 1976 (3) SA 226 (C).
6. Castell v De Greef 1994 (4) All SA 63 (C).
7. South Africa. National Health Act No. 61 of 2003.
8. Constitution of the Republic of South Africa, 1996.
9. Olejarczyk JP, Young M. Patient Rights And Ethics. StatPearls, 2021. https://www.ncbi.nlm.nih.gov/books/NBK538279 (accessed 16 September 2022).
10. Neff MJ. Informed consent: What is it? Who can give it? How do we improve it? Respir Care 2008;53(10):1337-1341.
11. World Medical Association. WMA Declaration of Helsinki - ethical principles for medical research involving human subjects. WMA, 2013.
12. Diemert DJ, Lobato L, Stycznski A, et al. A comparison ofthe quality of informed consent for clinical trials of an experimental hookworm vaccine conducted in developed and developing countries. PLoS Negl Trop Dis 2017;11(1):e0005327. https://doi.org/10.1371/journal.pntd.0005327
13. Appelbaum PS, Roth LH, Lidz CW, et al. False hopes and best data: Consent to research and the therapeutic misconception. Hastings Cent Rep 1987;17(2):20-24. https://doi.org/10.2307/3562038
14. Wilkins JM, Forester BP. Informed consent, therapeutic misconception, and clinical trials for Alzheimer's disease. Int J Geriatr Psychiatr 2020;35(5):430-435. https://doi.org/10.1002/gps.5262
15. Gota V, Nookala M, Yadav A, et al. Quality of informed consent in cancer clinical trials in India: A cross-sectional survey. Natl Med J India 2018;31(6):334-338. https://doi.org/10.4103/0970-258X.262900
16. Moloi GP. Informed consent: Communication and miscommunication in clinical trials. MCur dissertation. Stellenbosch: Stellenbosch University, 2012. [ Links ]
17. Varkey B. Principles of clinical ethics and their application to practice. Med Princ Pract 2021;30(1):17-28. https://doi.org/10.1159/000509119
18. Broude v Mclnosh and Another 1998 (3) SA 60 (SCA).
19. World Data Atlas South Africa. South African mean age of childbearing. https://knoema.com/atlas/SouthAfrica/topics/Demographics/Fertility/Age-of-childbearing (accessed 24 December 2021).
20. García-García EM, Sánchez-Sabater B, Medrano, et al. Sociodemographic factors affecting the comprehension of clinical information by inpatients undergoing trauma surgery. Rev Esp Cir Ortop Traumatol (Engl Ed) 2019;63(5):355-360. https://doi.org/10.1016/j.recot.2019.04.001
21. Burks AC, Keim-Malpass J. Health literacy and informed consent for clinical trials: A systematic review and implications for nurses. Nursing Res Rev 2019;9:31-40. https://doi.org/10.2147/NRR.S207497
22. Britz R, le Roux-Kemp A. Voluntary informed consent and good clinical practice for clinical research in South Africa: Ethical and legal perspectives. S Afr Med J 2012;102(9):746-748. https://doi.org/10/7196.SAMJ.5498
23. Reuters. Eastern Cape back to worst unemployment in SQA with nearly half without jobs. Reuters, 2021. https://www.dispatchlive.co.za/news/2021-08-25-sas-unemployment-rate-climbs-to-new-record-high-above-34/ (accessed 20 December 2021).
24. Alexa-Stratulat T, Neagu M, Neagu A-I, et al. Consent for participating in clinical trials - is it really informed? Dev World Bioeth 2018;18(3):299-306. https://doi.org/10.1111/dewb.12199
25. Cassell EJ. The Principles of the Belmont Report Revisited: How Have Respect for Persons, Beneficence and Justice Been Applied to Clinical Medicine? Hasting Cent Rep 30, no. 4. 2000:12-21. https://doi.org/10.2307/3527640
26. Wendler D. Must research participants understand randomization? Am J Bioeth 2009;9(2):3-8. https://doi.org/10.1080/15265160802654145
27. Appiah R. Gurus and Griots: Revisiting the research informed consent process in rural African contexts. BMC Med Ethics 2021;22(1):98. https://doi.org/10.1186/s12910-021-00659-7
 Correspondence:
Correspondence:
V C Nikodem
vcnikodem@gmail.com
Accepted 12 July2022














