Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Human Rights Law Journal
On-line version ISSN 1996-2096
Print version ISSN 1609-073X
Afr. hum. rights law j. vol.23 n.1 Pretoria 2023
http://dx.doi.org/10.17159/1996-2096/2023/v23n1a2
ARTICLES
A promising potential: Using the right to enjoy the benefits of scientific progress to advance public health in Africa
Remmy ShawaI, *; Fons CoomansII, **; Helen CoxIII, ***; Leslie LondonIV, ****
IFaculty of Health Sciences, Department of Public Health and Family Medicine, University of Cape Town, South Africa. https://orcid.org/0000-0001-9623-8272
IIDepartment of International and European Law, Maastricht University, The Netherlands. https://orcid.org/0009-0006-6734-1741
IIIDivision of Medical Microbiology and Institute of Infectious Disease and Molecular Medicine, University of Cape Town, South Africa. https://orcid.org/0000-0002-6538-7192
IVDivision of Public Health Medicine, University of Cape Town, South Africa. https://orcid.org/0000-0003-1297-2758
SUMMARY
In 2020 the United Nations Committee on Economic, Social and Cultural Rights published its 25th General Comment on the right to enjoy the benefits of scientific progress (REBSP). The General Comment describes the normative content of the right, including the obligations of the state and the entitlements of rights holders. It addressed the major gap in the REBSP, which was the lack of internationally-accepted interpretations of what the right entails. This article aims to shed light on the REBSP, and to demonstrate how it can be applied to advance public health. The article argues that the application of the REBSP requires a balancing act between the rights of researchers or scientists and the rights of users of the scientific knowledge they generate. It further argues that, when applied to health, the REBSP has the potential to improve access to better prevention, diagnosis and treatment of diseases, and could draw attention to neglected diseases, which mostly affect developing countries.
Key words: human rights; science; public health
1 Introduction
The International Covenant on Economic, Social and Cultural Rights (ICESCR) proclaims the right of everyone to 'enjoy the benefits of scientific progress and its applications' (article 15(1)(b)). However, until recently there was widespread uncertainty about the precise meaning of this right and its legal ramifications.1 In 2020 the Committee on Economic, Social and Cultural Rights (ESCR Committee) published General Comment 25 on science and economic, social and cultural rights. For the first time, the treaty body responsible for monitoring and guiding the implementation of economic, social and cultural rights provided its general interpretation of the right to enjoy the benefits of scientific progress (REBSP), marking the beginning of some form of international consensus on what this right entails, and the specifics around corresponding entitlements and obligations.
Suffice to note that this is only the beginning of what may be a long journey towards the full realisation of the REBSP. Realising the REBSP is difficult primarily because the right itself is complex and includes multiple spheres, such as science, intellectual property and international cooperation, and has far-reaching consequences for global politics and international trade. It is further complicated by the context in which the production of science takes place, often stretching beyond national borders and national jurisdictions,2thereby raising questions of extraterritorial obligations.
Access to the benefits of scientific progress in public health has received less attention than ensuring the protection of scientific discoveries. The latter has been a topic of thorough discussion and debate under intellectual property rights and law,3 which arguably has led to more attention being paid to scientific knowledge for the benefit of innovators.4 In the same way, public health problems of significant magnitude have been neglected or, if pursued, the scientific discoveries have been too costly to benefit the majority in need.5 A good example is the case of tuberculosis, which remains a major problem in poor countries, but has seen limited scientific progress. In fact, until 2012 there had not been any new treatment for tuberculosis in over 40 years.6
The article containing the REBSP in ICESCR has three parts: Paragraph 1(a) speaks to the 'right to participate in cultural life'; paragraph 1(b) speaks to the REBSP; and paragraph 1(c) speaks to the 'right to benefit from the protection of the moral and material interests resulting from any scientific, literary or artistic production of which a person is an author'. Paragraphs 1(b) and 1(c) often lead to tensions regarding human rights and intellectual property rights, respectively. Therefore, it is important that efforts to realise the REBSP maintain a balance of the two. This is to ensure that profits of the authors do not compromise the benefits of the users, or that benefits of users should not make it difficult for authors to reap benefits from their innovation. In fact, the second part of article 15, which speaks to rights of the author, does not apply to corporations, as the claim that corporations have rights is contested.7 On the other hand, corporations can rely on intellectual property rights found in the WTO Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement). However, when using the terms 'authors of science', 'researchers' or 'scientists', this article includes in general terms both institutions (such as corporations) and individual or independent researchers.
As a human right, the REBSP is a 'fundamental, inalienable and universal entitlement belonging to individuals and, under certain circumstances, groups of individuals and communities'.8 The premise is that because this is a human right, it is different from, and should not be superseded by, intellectual property rights which are essentially private ownership rights. However, there are arguments for and against intellectual property rights as human rights. Derclaye, for example, argues that there is no intrinsic conflict between human rights and intellectual property rights because intellectual property rights in fact are human rights,9 while others, including the ESCR Committee, argue that unlike human rights, intellectual property rights usually are temporary, can be 'revoked, licensed or assigned to someone else'. This article posits that there is a need for a balancing act where corporate behaviour is limited when it threatens human rights, under circumstances where intellectual property rights prevent people from accessing treatment or decent livelihoods.
In this article we seek to draw attention to the REBSP, a little-known but theoretically powerful right with substantial potential to impact positively on public health. We ask the question of how the REBSP can advance public health, and attempt to link this right to the elements of the right to health in ICESCR, as well as the ESCR Committee's interpretation of the right to health in General Comment 14. General Comment 25 on the REBSP in section two provides guidance into the normative content of the right, including its minimum core obligations on state and non-state actors, and the entitlements it presents on rights-holders. However, for the purposes of this article we limit our discussions to how the REBSP can advance public health, and do not discuss its normative content.
2 Right to enjoy benefits of scientific progress
The REBSP is considered an important right linked to the attainment of other social and economic rights, such as the right to health, food and technology.10 However, until recently it had not been well conceptualised,11 and lacked universal interpretation.12 Previous human rights discourse in science focused on ethics and doing no harm, but not necessarily advancing science for the benefit of people. Within the human rights framework, the REBSP brings to the fore not only elements of scientific advancement and its benefits, but also critical issues of legislation, such as adopting a framework law as a means of strengthening the implementation of the REBSP at the domestic level.
What makes the REBSP even more difficult to implement or monitor is its object and, consequently, its corresponding obligations. In most international human rights instruments, the REBSP seems to be aimed at addressing the following interests: (i) non-discriminatory access to the benefits of scientific progress and its applications;13 (ii) the opportunities for all to contribute to the scientific enterprise;14and (iii) the protection from adverse effects of science.15 These objects can be grouped into two distinct categories: the protection of the interests of producers of scientific knowledge (the authors); and protecting the interests of the beneficiaries (the users). However, these two interest groups do not always work together and, in many cases, their interests can be in opposition.
Much work has been done to understand and elaborate on both the benefits and potential dangers of science in society, although not particularly in the context of human rights. Despite the opportunity that the REBSP presents to look more closely at science and its relation to human rights, this particular right, which has been less theorised, only started to receive more attention in the early 2000s.16 Further, neither the Universal Declaration of Human Rights (Universal Declaration) nor ICESCR has any 'explicit formulation about the ideological or philosophical direction that science should take'17 or what is meant by scientific progress. As noted earlier, General Comment 25 of 2020 attempts to address the aspect of the REBSP most often neglected, which is access to scientific benefits.
Previously, where the REBSP had been interpreted, the emphasis was often placed on ensuring that science is not used to the 'detriment of human rights and freedoms and the dignity of the human person'.18
The same concerns were raised earlier at the World Conference on Human Rights, which cautioned against the possible adverse impact of biomedical sciences and technology on people's human rights.19This interpretation of the right is limiting because it appears to focus on the need to protect people from the negative effects of science, rather than seeing science as a means to advance the rights of people or, in this case, to improve people's health outcomes.
This new interpretation of the REBSP by the ESCR Committee makes a case for scientific progress, and access to benefits arising from such progress. Further, it provides many vulnerable populations, especially from developing countries, with the basis to demand the provision of better health interventions and medicines from their governments. With so many health challenges facing developing countries, including the COVID-19 pandemic, scientific progress in alleviating major causes of, or discovering cures for diseases, can potentially be utilised to save many lives. Currently, the world's poorest remain excluded from the benefits of scientific progress in many ways, or, as observed in efforts to provide COVID-19 vaccines, wealthier countries receive preferential treatment in accessing and enjoying scientific breakthroughs, simply through greater purchasing power.
3 Unpacking scientific progress and access to benefits
3.1 Scientific progress
Traditionally, science has been perceived as a study that seeks to discover new knowledge or to further existing knowledge about phenomena that occur in nature or society.20 The United Nations Educational, Scientific and Cultural Organisation (UNESCO) reports that 'every dollar invested in research and development (R&D) generates nearly two dollars in return', underscoring the importance of R&D in driving economic growth.21 While the use of science for economic development can indirectly contribute to public health and well-being, technological advancements such as the development of mechanical or biological weapons can adversely infringe on human rights.22 Even when the application of science is used for economic advancement, it can very easily fail to meet the minimum human rights requirements,23 such as equal and non-discriminatory access.
It is from R&D that new and better treatment regimens or prevention methods are discovered and, therefore, it is important to understand how scientific research plays out in modern societies. In practice, research can either be for profit or not, regardless of whether it is meant to add value to people's health. For example, research done by or with government support is often used for non-profit purposes, while private institutions engage in scientific research with future profit in mind. Some authors have argued that the two can co-exist and that the difference is not fundamental but based on the skills and interests of the researchers.24 It therefore is not uncommon for publicly-financed research to be used to advance largely private interests. Private researchers are able to apply and access government funding for their research, yet not every government has systems and protections in place to ensure that results of or benefits from such research actually benefit the public.25 Wouters and colleagues have demonstrated the extent to which public funding has underwritten the development of current vaccines for COVID-19, almost all of which have been patented under intellectual property protections afforded by patent law.26
3.2 Access to benefits (enjoying the benefits)
Regarding access to the benefits of scientific progress, states face a challenge in their obligation to respect the REBSP. This challenge comes from the complex nature and circumstances in which the production of science and its benefits occur.27 This is because science in itself is an unbounded or broad field that commonly does not occur within the confines of one country. This is also referred to as 'the 'universal' or 'global' nature of science.28 As a result, science's unlimited nature may conflict with the confined nature of human rights. Human rights by their nature need to be domesticated and applied within national jurisdictions29 because, in essence, human rights apply to the territories of states. And persons who are within the jurisdiction of a state are entitled to the protection of these rights. However, when the conduct of a state has negative effects on the enjoyment of the rights of people living in another country, such a state may be bound by its human rights obligations outside its territory (extra-territorial obligations).
Furthermore, a state may be required to regulate the foreign conduct of multinational companies that are domiciled in its territory.30 For example, South Africa, being a country with a high prevalence of HIV, tuberculosis and COVID-19, and a strong research infrastructure, has become a destination for HIV, tuberculosis and COVID vaccine and treatment trials. It is important to consider the interplay between such research occurring in South Africa and the obligations of the countries in which the pharmaceutical companies are domiciled. Also, the responsibilities of the companies themselves are crucial in understanding access to the benefits of science.
A classic example of the conflict between the unbounded nature of science and the bounded nature of human rights is the case of the patent battle involving South Africa, India and the United States in the manufacturing of generic drugs for HIV.31 On one hand, South Africa and India wished to fulfil the right to health for their citizens by manufacturing generic drugs and improving access to low-cost drugs (the bounded nature of human rights). On the other hand, the production of science (generic drugs) had far-reaching implications that involved international and national pharmaceutical companies, and international agencies such as the World Trade Organisation (WTO) (the unbounded nature of science). This is a conflict between asserting the intellectual property rights of some versus the right to benefit from scientific progress for others or, to put it simply, balancing between the right of the authors, and that of the users of science.
With this conflict in mind, the application of the REBSP cannot simply end at domestic laws and policies, but also needs to extend to extraterritorial obligations, specifically, how extraterritorial obligations can shift the relationship between human rights and science. Simply defined, extraterritorial obligations are
obligations relating to the acts and omissions of a state within or beyond its territory, that have effects on the enjoyment of human rights outside of that state's territory; and obligations of a global entity that are set out in the Charter of the United Nations and human rights instruments to take action.32
3.3 REBSP potential for public health in Africa
The discourse on the relationship between health and human rights has received much attention in recent years. Previously, health policies were developed without much consideration of their impact (positive or negative) on human rights. Similarly, the human rights community seldom engaged in public health discourse or scholarship. However, through a considerable body of scholarship over the last two decades,33 it has become clear that there is a very strong relationship between health and human rights, from three points of view as seen in figure 1: (i) the impact of health policies on human rights; (ii) the impact of human rights on health; and (iii) the inextricable link between the protection and promotion of health on the protection and promotion of human rights.34 The discussion on access to health care as a human right is one of the ways in which health and human rights are linked and has led to the strengthening of the conceptualisation and implementation of the right to health.
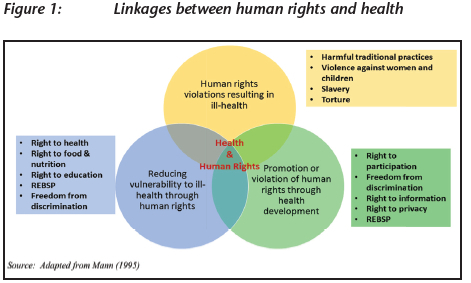
The constitution of the WHO defines health as a 'state of complete physical, mental, and social wellbeing, and not merely the absence of disease or infirmity'.35 Health is not limited to disease prevention and access to healthcare services, but includes the social and economic conditions necessary for people to enjoy good health. The Alma Ata Declaration on Primary Health Care adopted at the World Health Assembly in 1978 states that health is a 'social goal whose realisation requires the action of many other social and economic sectors in addition to the health sector'.36 Some underlying social determinants of health include 'access to clean water, sanitation, food, nutrition, housing, healthy occupational and environmental conditions, education, information, decent work, and livelihood'. These social determinants of health are explicitly spelled out in the framing of the right to health in the Universal Declaration and ICESCR.
ICESCR expands on the right to health in article 12, where it states that 'the State Parties to the present Covenant recognize the right of everyone to the enjoyment of the highest attainable standard of physical and mental health'. ICESCR articulates some of the steps that states need to take to ensure the full realisation of the right to health. These steps include 'reduction in infant and child mortality, environmental and industrial hygiene; prevention, treatment and control of epidemics; and provision of medical services'.37
Despite progress made in the conceptualisation and application of the right to health, inequalities in public health persist. The glaring differences between health outcomes of the rich and the poor within countries, or between low and high-income countries, are evidence of inequality in health outcomes.38 Health inequality not only presents in the form of inequitable access to health care and other social services critical to health, but also presents itself in the different types of diseases experienced by the rich and the poor. For example, diseases such as tuberculosis are prevalent in low-income populations that experience poor housing and living conditions. Despite the large numbers that such 'diseases of the poor' affect, attention to these diseases is often scant.39 These health problems are less likely to see scientific progress in the development of innovative ways to eliminate them. In similar ways, COVID-19 has highlighted these differences between wealthier and poor countries, where the race towards access to a vaccine was dominated by high-income countries.
As of April 2021 only 4 per cent of the global population had received at least one dose of the COVID-19 vaccine.40 Vaccine coverage is higher among developed countries, with Africa having less than 1 per cent coverage. On the other hand, North America and Europe have achieved vaccine coverage of above the global average, with 21,09 and 13,57 per cent respectively.41 While this data refers to actual vaccine coverage, most developed countries have already pre-ordered vaccines that are in development. An estimated 11 billion doses of the COVID-19 vaccine are needed globally to be able to inoculate 70 per cent of the world's population, based on the assumption that a person would need two doses. As of April 2021, orders of over eight billion doses had been confirmed, but six billion of these doses have already been reserved by developed countries, while middle-income and low-income countries have only secured 670 million doses (less than one-third),42 although developing countries account for 80 per cent of the world's population. How, then, should scientific progress be understood and how can the REBSP contribute to better health outcomes for the poor?
The link between the REBSP and public health has in recent years sparked interest from public health and human rights scholars.43Access to medicines has been at the centre of this interest, highlighting the need to look beyond investing in research and development, and to ensure that R&D is translated into knowledge and products accessible to those who need them. Human rights activists and health practitioners, however, have battled with skewed market mechanisms and little investment in research and development for diseases that predominantly affect poor countries. Inadequate and, in some cases, sheer lack of investment in diseases affecting the poor results in a lack of effective health technologies such as vaccines, drugs and diagnostics.44 Despite an increase in the protection of intellectual property rights through patents, markets have failed to fill the gap since patents are not an effective incentive to invest in diseases that affect the poor who generally cannot afford to pay for their medicines.45
In Africa, the REBSP is intricately connected to public health in several ways. First, in order for people to enjoy good health, it is necessary to continuously invest in newer and more effective scientific research that can make health and its social determinants accessible and affordable to everyone, particularly the most vulnerable. While progress has been made in the health status of sub-Saharan African countries, the health status of the region remains one of the worst in the world.46 In this respect, the REBSP could become a vehicle for facilitating public health. Second, the REBSP has implications on how scientific advancements are produced, shared and utilised. Because authors of scientific knowledge, if they are corporations, are protected through intellectual property rights, the REBSP can assist in making sure that these rights are not realised at the expense of the human rights of the users. The REBSP becomes a significant mediator between a human right (the right to health) and a property right (intellectual property right). This is important considering that intellectual property rights should support, and not hinder, human rights.47
Further, to appreciate the REBSP, and its role in advancing public health, one ought to turn to the tenets of the right to health as presented in international human rights law. Article 12 of ICESCR recommends four steps that the state can take to realise the right to health. By taking a closer look at these proposed steps, it is clear to see how the REBSP is essential to facilitating public health in that, for all these steps to be successful, scientific progress becomes indispensable in more ways than one. Below, we highlight the four steps highlighted in ICESCR that states need to take, and show how the REBSP is critical to each one of them.
3.3.1 The reduction of stillbirth rate and infant mortality
Preventing stillbirth and infant mortality requires, among other things, the development of new vaccines to prevent diseases that affect infants, better pregnancy monitoring and care to prevent pre-term or post-term births, and earlier detection of complications; in other words, more effective diagnostic techniques. Technology is also key to enhancing prevention efforts, as has been found in South Africa where the government has introduced mHealth strategies such as MomConnect. MomConnect is an initiative by the South African National Department of Health aimed at supporting and promoting maternal and child health by way of mobile technology. mHealth is the use of 'mobile computing, medical sensor, and communications technologies used for the delivery of health-related services and the support of medical and public health practice'.48 All these measures require science and R&D. For example, the discovery of and access to vaccines have significantly reduced the burden of disease caused by measles, polio and tetanus, and eradicated smallpox, demonstrating the role of science and R&D in reducing child mortality and morbidity.
3.3.2 Environmental and industrial hygiene
The prevention of occupational and environmental disease requires science to detect and control hazards. Methods to rehabilitate asbestos dumps, for instance, require research to identify the safest and most cost-effective options. Similarly, science is used to generate better tools to screen employees at workplaces for early detection of lung impairment arising from exposure to workplace hazards. By using science to detect and mitigate environmental hazards, employers would be safeguarding the health and well-being not only of their employees, but also of the communities around them. In this regard, the REBSP becomes critical to the advancement on public and environmental health.
3.3.3 Prevention, treatment and control of epidemic, endemic, occupational and other diseases
Science can prevent, treat and control epidemics, and yield new knowledge to develop vaccines, explore more effective public health practices, and develop more effective treatments and ways of managing diseases. The COVID-19 pandemic has highlighted this invaluable contribution of science to public health. Unfortunately, even when high quality science exists, the acceptance and application of such scientific knowledge are marred by politics and personal agendas,49 as it has been observed through the emergence of antivaccine groups across the globe. However, this does not take away the fact that the conduct of quality science is crucial in protecting public health from such global pandemics.
3.3.4 Assurance of medical service and attention in the event of sickness
Science assures medical service and medical attention from the most basic medical service to more complex ones. The use of ambulances, which are now equipped with advanced medical technology, is a result of scientific research. In South Africa, for instance, a community-based mobile clinic model scored successes in delivering treatment for HIV to adolescent girls and young women.50 Similarly, to facilitate better health care, healthcare facilities need to be equipped with modern technology, from treating common illnesses, to more uncommon ones. The magnetic resonance imaging (MRI), for instance, is a crucial part of health, and although it is still costly, demonstrates the possibility that scientific advancement can bring to public health and well-being. Similarly, the use of digital X-ray technology has significantly improved diagnosis of tuberculosis and increased its early detection.
3.4 REBSP beyond borders
Although only now being strengthened in international law, the REBSP in fact is very complex as it requires not only a specific state to respect, protect and fulfil it, and not only in its territory, but also through international cooperation and, where necessary, extraterritorial obligations by one state in another territory or country. Because resources vary from one state to another, there are huge disparities between states in the implementation of this right, particularly between developed and developing countries. This calls for the need to clearly define the REBSP and situate state obligations within a global political economy in which both state and non-state actors have significant influence over the laws, policies, and economies. This is why ICESCR in categorical terms demands 'international assistance and cooperation'.
Thus, the legislative obligations on governments are not only to adopt a framework law as a means of strengthening the implementation of the REBSP at the domestic level, but also relate to the transfer of scientific knowledge to benefit developing countries, pursuant to the Development Agenda Recommendation 25 of the World Intellectual Property Organisation (WIPO). The Recommendation emphasises the need to
promote the transfer and dissemination of technology, to the benefit of developing countries and to take appropriate measures to enable developing countries to fully understand and benefit from different provisions, pertaining to flexibilities provided for in international agreements, as appropriate.51
Therefore, the failure to respect, protect or fulfil the REBSP cannot be limited to prevailing social or economic challenges but should include the state's own actions, omissions and accountability under international and national law. Further, the TRIPS Agreement in article 66(2) requires developed countries to provide incentives to institutions in their territories so as to promote and encourage knowledge and technology transfer to least developed member states.
The most promising and widely-used Coronavirus vaccines were developed by institutions domiciled in developed countries. These include the Moderna vaccine by US institutions; the Pfizer-BioNTech vaccine by a German and a US company; the Johnson&Johnson vaccine by a US company; and the Oxford-AstraZeneca vaccine by UK companies. Therefore, one may argue that in accordance with the TRIPS Agreement, developed countries, among them the US, UK and European Union (EU) countries, have a responsibility to facilitate the transfer of benefits from the scientific advancement in COVID vaccines to developing and least developed countries. Although some developed countries have pledged to donate or have already donated to the COVAX52 facility, a global suspension of patents on all COVID vaccines until the pandemic is under control would be a game changer.53
South Africa and India have tabled a patent waiver proposal for COVID-19 vaccines, medicines, diagnostics, and medical technologies at the WTO TRIPS Council. The proposal seeks to secure patent reprieves on COVID-19 vaccines by developers, in order to allow the production of generic products. However, while developing and least developed countries are in support of the proposal, some developed countries are still opposed to this proposal, making it highly unlikely that a consensus in the affirmative will be reached.54 This may be interpreted as developed countries' failure to facilitate the transfer of both knowledge and products to least developed countries. This inaction amounts to non-compliance with article 66(2)55 of the TRIPS agreement.56
Just as in the case of any international human right, the enforceability of the REBSP depends largely on the domestication of the right at the national level through legal and judicial measures. These measures are not limited to adopting the right in the national constitution, but may also include the development of new, and the enforcement of existing policies and acts, including a clear framework law for the domestic application of the right. However, a lack of clarification of both normative entitlements and obligations has hindered the domestication of the REBSP and presents a difficulty in measuring a violation. This is why the articulation of the REBSP in the 2020 General Comment 25 by the ESCR Committee presents a very significant leap towards a globally-recognised right, with clear obligations and entitlements.
In terms of unpacking cultural rights (article 15 of ICESCR) only one dimension seems to receive much attention, namely, the right of a person to benefit from ideas of which he (sic) is the author (article 15(c)) and having the freedom to engage in scientific discoveries. Without a similar emphasis on the rights of the user, or beneficiary of scientific progress and discoveries, it becomes difficult to use this right to advance public health. This bias towards implementing only article 15(c) on the rights of authors negatively affects the human rights of the population and compromises their access to the benefits of scientific progress critical to the enjoyment of their health.
Framing problems of access to diagnosis as a human rights issue would require a strong linkage between an argument for enhancing access to medicines and the state's mandate in both national and international human rights law. Currently, this linkage exists mainly through the right to health. However, even within the rights framework, a rights-based approach to health must not only look at the right to health but also at other rights that have implications for health. This is relevant based on the principles of the indivisibility and interdependence of rights, for within the rights-based approach, discriminatory treatment disparities have already been framed as 'rights violations', and the REBSP broadens the claims and suggests that the state, as well as non-state entities, must bear the responsibility.
Having a right is no guarantee that the right will be realised, nor does the REBSP guarantee good public health. However, with this responsibility should come corresponding measures of accountability that allow scrutiny of the efforts of government in the realisation of the right. If well conceptualised, the international norms relating to the REBSP would assist in developing measures of accountability for both governments and non-state actors such as transnational corporations.
4 Conclusion
For public health to be safeguarded, states need to meet their obligations under article 12(2)(c) of ICESCR - which include the prevention, treatment and control of epidemics and occupational diseases. In this regard, scientific progress is expected to result in new, advanced and better ways of providing for health needs such as epidemic prevention or treatment. It is, after all, scientific research that has led to breakthroughs in managing diseases from prevention to diagnosis, to treatment. In the case of tuberculosis, for example, the contribution that investment in tuberculosis research brings includes advances in better preventive treatment, enhancing timely access to current treatment through better diagnostics and improved treatment regimens for drug-resistant tuberculosis. Scientific research also has the potential to discover safer, more effective and affordable medicines to prevent and treat different diseases, including COVID-19.
For the REBSP to bring about meaningful impact in public health, there needs to be deliberate systems and strategies to ensure that both the rights of authors of science and the users are protected. However, should a conflict arise between these two categories, the rights of users, being human rights, should be prioritised over the rights of authors to their intellectual property. Importantly, a global discourse is required to re-evaluate the place of profits and global markets in public health and re-imagine a world where public health research becomes driven more by public health needs than by the need for profit. Political and legal debates around the human rights obligations of transnational corporations currently are ongoing, to clarify the role of business in realising human rights.57
The REBSP is not a novel right. It has long been enshrined in various regional human rights treaties and in two of the most fundamental human rights instruments, namely, the Universal Declaration and ICESCR. Further, the REBSP is closely related to other human rights enshrined in ICESCR, such as the right to health' and, therefore can facilitate and accelerate the realisation of these rights, as a 'facilitatory' and an 'enabling' right. For example, by pursuing the REBSP in public health, such as developing better medical technologies and affordable treatment, states would also be ensuring the realisation of the right to health. Similarly, many other rights would be better realised if there was adequate scientific advancement, for the benefit of communities and populations.
Having outlined the evident potential of the REBSP in public health, we submit that it is high time that international agencies, both public and private, paid more attention to the REBSP, and use it to guide the discourse around access to medicines for neglected diseases in poorly-resourced communities. The General Comment on the REBSP has created a starting point for this this level of international attention, but there now is a need for further application of the right through national laws.
* BA (Zambia) MPH PhD (Cape Town); remmyshawa@gmail.com
** BA MA (Amsterdam) PhD (Maastricht); fons.coomans@maastrichtuniversity.nl
*** BSc MPH PhD (Melbourne); helen.cox@uct.ac.za
**** MB ChB MMed Public Health (Cape Town) DOH (Witwatersrand) BSc Hons (Stellenbosch) MD (Cape Town); Leslie.london@uct.ac.za
1 JM Wyndham & M Weigers 'The right to science - Whose right? To what?' (2015) 4 European Journal of Human Rights 431. [ Links ]
2 S Besson 'Science without borders and the boundaries of human rights: Who owes the human right to science?' (2015) 4 European Journal of Human Rights 462. [ Links ]
3 As above.
4 G Yamey 'Excluding the poor from accessing biomedical literature: A rights violation that impedes global health' (2008) 10 Health and Human Rights 21. [ Links ]
5 As above.
6 S Tiberi et al 'The challenge of the new tuberculosis drugs' (2017) La Presse Médicale 46. [ Links ]
7 A Kulick 'Corporate human rights?' (2021) 32 European Journal of International Law 537. [ Links ]
8 ESCR Committee 'General Comment 17: The right of everyone to benefit from the protection of the moral and material interests resulting from any scientific, literary or artistic production of which he or she is the author' (2006).
9 E Derclaye 'Intellectual property rights and human rights: Coinciding and cooperating' (2008) Common Market Law Review https://ssrn.com/abstract=1116010 (accessed 20 March 2023).
10 S Besson 'Introduction - Mapping the issues' (2015) 4 European Journal of Human Rights 403-410.
11 L Shaver 'The right to science: Ensuring that everyone benefits from scientific and technological progress' (2015) 4 European Journal of Human Rights 411.
12 AR Chapman 'Towards an understanding of the right to enjoy benefits of scientific progress and its applications' (2009) 8 Journal of Human Rights 1-36.
13 Shaver (n 11) 411-431.
14 Besson (n 2) 462-486.
15 Human Rights Council 'Report of the Special Rapporteur in the field of cultural rights' (2012) A/HRC/20/26 1-24.
16 Y Donders 'The right to enjoy benefits of scientific progress: In search of state obligations in relation to health' (2011) 14 Medicine, Health Care and Philosophy 371-381.
17 WA Schabas 'Looking back: How the founders considered science and progress in their relation to human rights' (2015) 4 European Journal of Human Rights 504-519.
18 Chapman (n 12) 1-36.
19 OHCr 'Vienna Declaration and Programme of Action' adopted by the World Conference on Human Rights in Vienna on 25 June 1993.
20 J Sellin & F Coomans 'Extraterritorial human rights obligations and the transfer of technology for local production and research and development for essential medicines' (2016) Maastricht Faculty of Law Working Paper 2016/7.
21 UNESCO Institute for Statistics 'Global Investment in R&D' (2017).
22 Chapman (n 12) 1-36.
23 As above.
24 C Carraro & D Siniscalco 'Science versus profit in research' (2003) 1 Journal of the European Economic Association 576.
25 OJ Wouters et al 'Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment' (2021) 397 The Lancet 1023.
26 As above.
27 J Rahko 'Internationalisation of corporate R&D activities and innovation performance' (2015) 25 Industrial and Corporate Change 1019.
28 Besson (n 2).
29 European Commission for Democracy Through Law 'Venice Statement on the Right to Enjoy Benefits of Scientific Progress and its Applications' (2009).
30 Besson (n 2); ESCR Committee General Comment 24 (2017) on state obligations under the International Covenant on Economic, Social and Cultural Rights in the context of business activities.
31 H Hestermeyer Human rights and the WTO: The case of patents and access to medicines (2007).
32 Sellin & Coomans (n 20); see also Maastricht Principles on the Extraterritorial Obligations of States in the Area of Economic, Social and Cultural Rights (2011) www.etoconsortium.org (accessed 20 March 2033).
33 EK Mpinga et al 'Health and human rights: Epistemological status and perspectives of development' (2011) 14 Medicine, Health Care and Philosophy 237.
34 J Mann et al 'Health and human rights' (1994) 1 Health and Human Rights 6.
35 FP Grad 'The preamble of the constitution of the World Health Organisation' (2002) 80 Bulletin of the World Health Organisation 981.
36 Declaration of Alma-ata 'International Conference on Primary Health Care' (1978) 6 USSR 12.
37 International Covenant on Economic, Social and Cultural Rights, 16 December 1966, United Nations, Treaty Series, vol 993.
38 World Health Organisation 'Closing the gap in a generation: Commission on Social Determinants to Health' (2008).
39 P Hotez & B Pecoul 'Manifesto for advancing the control and elimination of neglected tropical diseases' (2010) 4 PLoS Neglected Tropical Diseases e718.
40 M Shrotri et al 'An interactive website tracking COVID-19 vaccine development' (2021) 9 Lancet Global Health E590.
41 Coronavirus (COVID-19) Vaccinations, https://ourworldindata.org/covid-vaccinations (accessed 7 April 2021).
42 'It's time to consider a patent reprieve for COVID vaccine' (2021) 592 Nature Editorial 7, https://doi.org/10.1038/d41586-021-00863-w (accessed 7 April 2021).
43 L London et al 'Multidrug-resistant TB: Implementing the right to health through the right to enjoy benefits of scientific progress' (2016) 18 Health and Human Rights: An International Journal; Sellin & Coomans (n 20); Donders (n 16) 371.
44 London et al (n 43).
45 J Lanjouw 'A patent policy proposal for global diseases' (2006) 1 Innovations: Technology, Governance, Globalisation 108-114.
46 D Sanders et al 'Public health in Africa' (2003) 1 Global Public Health: A New Era 135.
47 G Rajvanshi & R Gupta Intellectual property rights vs human rights: A need to re-examine the relationship between the two to enhance social being (2011).
48 South African National Department of Health 'Health Strategy 2015-2019'.
49 RH Brown & EL Malone 'Reason, politics, and the politics of truth: How science is both autonomous and dependent' (2004) 22 Sociological Theory 106.
50 E Rousseau 'A community-based mobile clinic model delivering PrEP for HIV prevention to adolescent girls and young women in Cape Town, South Africa' (2021) 21 BMC Health Services Research 1-10.
51 World Intellectual Property Organisation - An Overview (2007.
52 COVID-19 Vaccines Global Access, abbreviated as COVAX, is a global initiative aimed at equitable access to COVID-19 vaccines led by UNICEF, Gavi, the Vaccine Alliance, the World Health Organisation, the Coalition for Epidemic Preparedness Innovations, and others.
53 F Coomans 'Responding to COVID-19: The extraterritorial human rights obligations perspective' (2020), https://gchumanrights.org/preparedness/article-on/responding-to-covid-19-the-extraterritorial-human-rights-obligations-perspective.html (accessed 14 May 2023).
54 K Zaman 'The proposal to the WTO for a new patent waiver on COVID-19 Vaccines and pharmaceuticals: Is it necessary under TRIPS?' (2021) 43 European Intellectual Property Review 645.
55 Art 66(2) of the TRIPS Agreement instructs developed country members to incentivise domestic enterprises and institutions 'for the purpose of promoting and encouraging technology transfer to least-developed country members'.
56 A Nawarat 'Exploring the COVID-19 vaccine IP waiver proposal at the WTO', https://www.pharmaceutical-technology.com/features/wto-ip-waiver-proposal-covid19-vaccine/ (accessed 13 April 2021).
57 P Werhane 'Corporate moral agency and the responsibility to respect human rights in the UN Guiding Principles: Do corporations have moral rights? (2016) 1 Business and Human Rights Journal 5-20.














