Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.49 n.4 Pretoria Oct. 2023
http://dx.doi.org/10.17159/wsa/2023.v49.i4.4029
REVIEW
Antibiotics in water bodies, cyanobacterial toxicity and odorous compounds release: a review
Bruna de Lemos NovoI, II; Fernanda Arruda Nogueira Gomes da SilvaIII; Luiz Carlos BertolinoII; Lidia YokoyamaI
ISchool of Chemistry, Federal University of Rio de Janeiro, 149 Athos da Silveira Ramos Avenue, University City, Rio de Janeiro, RJ, Brazil
IICenter for Mineral Technology/CETEM-MCTI, 900 Pedro Calmon Avenue, University City, Rio de Janeiro, RJ, Brazil
IIIInstitute of Chemistry, Federal University of Rio de Janeiro, 149 Athos da Silveira Ramos Avenue, University City; Rio de Janeiro, RJ, Brazil
ABSTRACT
The present study aimed to propose a new cause of odorous compounds release, i.e., the presence of antibiotics in water bodies and its toxicity to cyanobacteria, known to be the main producer of geosmin (GEO) and 2-methylisoborneol (2-MIB). Therefore, a literature review was carried out regarding the problems caused by antibiotics in aquatic environment, including cyanobacterial blooms and GEO and 2-MIB release. In addition, a bibliometric analysis was performed using the VOSviewer software based on the results obtained from the Web of Science (WOS) database. This review aims to build a scientific understanding of the problem, presenting interesting points that converge with the proposed association. It is worth mentioning that no work has been found in the literature that has proposed this relationship. Thus, based on the bibliographic survey, observations and information acquired in recent years about cyanobacterial blooms and environmental contamination by pharmaceutical drugs, one of the main causes of an earthy and musty flavour and odour in a drinking water supply is the toxicity imposed by the presence of antibiotics in aquatic environments on cyanobacteria.
Keywords: cyanobacterial blooms, antibiotics, toxicity, geosmin, 2-methylisoborneol
INTRODUCTION
Worldwide, several studies point to the risk that cyanobacterial blooms can cause to the environment (Carmichael, 2001; Paerl and Huisman, 2008; Paerl et al., 2011; Paul and Otten, 2013; Huisman et al., 2018; Zhang et al., 2020). Microalgae and cyanobacteria occupy trophic levels within food webs, and changes in their diversity and abundance could have an indirect but significant effect on the rest of the freshwater community (Pan et al., 2008), such as the release of cyanotoxins and odorous compounds like geosmin (GEO) and 2-methylisoborneol (2-MIB). For this reason, they have been highlighted as suitable organisms for environmental toxicity assessment (Holten- Lutzh0ft et al., 1999; Andreozzi et al., 2004; Pan et al., 2008, Rico et al., 2018).
Studies show that the main causes of cyanobacterial blooms are an eutrophic medium (Paerl and Huisman, 2008; Liu et al., 2012; Paerl and Otten, 2013; Huisman et al., 2018; Pham et al., 2020), rising CO2 levels (Paerl et al., 2011; Huisman et al., 2018), rainy season (Paerl and Otten, 2013; Pham et al., 2020; Zhang et al., 2020) and higher water temperatures due to global warming (Paerl and Huisman, 2008; Paerl et al., 2011; Paerl and Otten, 2013; Huisman et al., 2018; Rico et al., 2018; Lee et al., 2020). However, there had been an increase in the number of studies that focus on the problem of improper disposal of antibiotics in aqueous mediums, causing a rise in bacterial resistance (Hirsch et al., 1999; Halling-S0rensen et al., 2000; Mog et al., 2020; Zhang et al., 2020), and subsequent damage not only to fauna and flora, but also human health. This is because conventional water treatment processes are not sufficient for the effective removal of these drugs (Bueno et al., 2012), which are found in low but significant concentrations in the domestic water supply (Kümmerer et al., 2009; Guo et al., 2016). Besides that, several studies point to the toxicity that antibiotic presence causes to algae and cyanobacteria, causing them to produce cyanotoxins (Liu et al., 2012; Huisman et al., 2018), which are responsible for liver, digestive and neurological diseases when ingested (Carmichael, 2001; Merel et al., 2013).
Lee et al. (2020), for example, observed in their study of a sample from a water reservoir in Korea under cyanobacterial bloom that the number of cyanobacteria was positively correlated with geosmin concentration, indicating that cyanobacteria are potentially the main source of this substance. Thus, it is possible that the presence of antibiotics in aqueous media, when associated with certain aquatic and climatic environmental factors, induce the production of odorous compounds giving flavour and odour to the water supply.
Thus, this review presents a concise assessment of available evidence in reference to antibiotic toxicity to algae and cyanobacteria, which results in harmful blooms, with the production of GEO and 2-MIB, threatening the environment and consequently society. To investigate this proposal, bibliometric research was carried out using the VOSviewer software based on the results of a search in the Web of Science (WOS) database. To our knowledge, no published work to date has pointed out this possible relationship and cause.
METHODOLOGY
Bibliometric analysis
Bibliometric analysis was performed as described by Van Eck and Waltman (2010), who were responsible for the development of VOSviewer, a program (available free of charge) for the creation and visualization of bibliometric maps. Through this platform it is possible, for example, to build maps based on the co-citation of data as well as the co-occurrence of keywords. To this end, a bibliographic search was carried out in the databases supported by the program (Web of Science, Scopus, Dimensions, Lens and PubMed), using keywords of interest. On completion of the search, it is possible to export the result and then use the software to generate bibliometric maps.
For our bibliometric analysis, the Web of Science collection was used as a database. Three bibliographic searches were carried out, aiming to cover the three main points of interest of this work: antibiotics, cyanobacterial blooms, and odoriferous compounds. For each of these points, three keywords have been selected, as shown in Table 1. It can be seen that the word 'cyanobacteria' is present as a keyword in all three searches. This is because it was the main link proposed between antibiotics and odorous compounds.
All three searches were performed by filtering the presence of keywords in all collection fields, in the past 10 years of publication, from 1 January 2012 to 18 August 2022. The results were exported in tab-delimited format and the 'Full Record' and 'Cited References' options were selected, as directed in the program manual (Van Eck and Waltman, 2022).
Finally, in the VOSviewer program, maps based on Web of Science bibliographic data were created. The type of analysis selected was cooccurrence, with all keywords as units of analysis. The full counting mode has been used as the counting method. The minimum number stipulated for the occurrence of keywords was equal to 5.
For the three maps, network visualization was selected. In this type of visualization, Items (such as keywords, for example) are determined by labels and circles. The size of the label and circle of an item are determined by the weight of the item: the higher the weight, the larger the label and circle. The colour of an item is determined by the cluster to which the item belongs. Lines between items represent links. The stronger the link between two items, the thicker the line used to display the link in the visualization of the currently active map (Van Eck and Waltman, 2010; Van Eck and Waltman, 2022).
LITERATURE REVIEW
Antibiotic toxicity to cyanobacteria
Antibiotics are natural, synthetic, or semi-synthetic compounds, which can kill or inhibit growth or metabolic activity of microorganisms. These compounds are biologically active molecules with antibacterial, antifungal, and anti-parasitic activities deliberately designed as a medicine to treat bacterial infections in both people and animals, and as feed additives or disease prevention in animal husbandry (Kovalakova et al., 2020).
Data obtained by the World Health Organization indicate that, from 2016 to 2018, for every 1 000 inhabitants the overall consumption of antibiotics ranged from approximately 4 to 64 daily doses (WHO, 2018). Studies indicate that the concentration of these drugs in wastewater is in the order of |ig to ng-L-1 (Hirsch et al., 1999; Kümmerer, 2009; Bengtsson-Palme and Larsson, 2016; Chow et al., 2021). However, with the intensification of consumption, there is also an increase in its disposal, which when carried out inappropriately or illicitly, generates serious damage to the environment.
Thus, antibiotics are regarded as emerging 'pseudo-persistent' environmental pollutants because of their continuous input (through high consumption and its various disposal routes, such as urine and faeces, hospital, and pharmaceutical industrial waste) and persistence in aquatic ecosystems (due to ineffective removal via conventional water treatment processes) (Ye et al., 2017).
According to Ding and He (2010), Roose-Amsaleg and Laverman (2015) and Kovalakova et al. (2020), long-term exposure to antibiotics can change microbial communities, the effects of which include variation in biogeochemical cycling, alteration of phylogenetic structure, expansion of resistance, and disturbance of ecological function in the micro-ecosystem.
Due to the high sensitivity of different kinds of algae to the presence of antibiotics in aqueous media, they are widely used as test organisms for environmental risk assessment, with cyanobacteria being the most sensitive (Holten Lutzh0ft et al., 1999; Andreozzi et al., 2004; Pan et al., 2008, Válitalo et al., 2017; Rico et al., 2018; Kovalakova, et al., 2020). This sensitivityt is due to the similarity of their physiological characteristics to those of the target bacteria for which antibiotics were designed (Van der Griten et al., 2010).
Several studies have pointed to the toxicological effect of antibiotics on algae (Holten Lutzh0ft et al., 1999; Halling-S0rensen, 2000; Andreozzi et al., 2004; Pan et al., 2008). The results obtained by Holten-Lutzh0ft et al., (1999) and Halling-Sorensen (2000) revealed a high toxicity to Microcystis aeruginosa, a freshwater cyanobacterium, from the tested antibiotics (amoxicillin, flumequine, oxolinic acid, oxytetracycline hydrochloride, sarafloxacin hydrochloride, sulfadiazine, and trimethoprim).
Andreozzi et al. (2004) investigated the toxicological effect of amoxicillin on the algae Chlorophyceae Psudokirkneriella subcapitata, Closterium ehrenbergii, Cyclotella meneghiniana and Cyanophyte Synechococcus leopoliensis, and the results pointed to a high toxicity for the blue-green algae. In addition, the results obtained by Pan et al. (2008) also revealed that amoxicillin severely inhibited the photosynthesis of cyanobacterium Synechocystis sp.
According to Kovalakova et al. (2020), in their research it was observed that the antibiotic amoxicillin has low toxicity for algae and bacteria, but it is extremely toxic, along with ciprofloxacin and ofloxacin, for cyanobacteria. However, assessing the real environmental risk of antibiotics to cyanobacteria requires a longer exposure time due to its slower growth when compared to algae.
González-Pleiter et al. (2017) studied intercellular free Ca2+ signals in cyanobacteria, since these appear to be a biomarker of exposure to pollutants, among them, antibiotics. The results showed that all the antibiotics studied induced a calcium transient for cyanobacteria, within the first moments of injection, indicating that antibiotics are harmful to this species. According to Válitalo et al. (2017), Microcystis aeruginosa is sensitive to amoxicillin, whereas Anabaena sp. CPB4337 can tolerate high concentrations of the same antibiotic. Of all the antibiotics tested, only trimethoprim was non-toxic to all cyanobacteria tested.
Ding and He (2010) explain that the inherent reason for these differences in toxicity is that antibiotics in general, even those designed to be broad-spectrum drugs, have selective effects on various groups of microbes. Besides that, Guo et al. (2016) attributes these differences to differences in antibiotic uptake, in the binding pockets in primary targets, in antibiotic elimination, and in active efflux pumps.
According to the results obtained by Dias et al. (2015), the response of freshwater cyanobacteria to antibiotics depends on the type/concentration of the antibiotic as well as cyanobacteria isolate, since they observed a strong decrease in cyanobacterial growth at the lowest antibiotic concentration (6-lactams, in particular amoxillicin); a decrease in cell viability at middle/ higher antibiotic concentrations (aminoglycosydes, tetracycline, and norfloxacine) and no effect at any antibiotic concentration (nalidixic acid and trimethoprim).
According to Liu et al. (2012), at certain concentrations, amoxicillin can stimulate cyanobacterial growth. In another study by Liu et al. (2014), the results obtained indicated that high levels of nitrogen associated with high levels of amoxicillin work together not only for cyanobacterial growth, but also for the production of toxins (microcystins), and the coexistence of these contaminants with cyanobacteria represents a major threat to the aquatic environment, requiring great attention, especially during cyanobacterial bloom periods.
Wan et al. (2015) observed that erythromycin can inhibit cyanobacterial growth at high levels, but stimulate their growth at relatively low concentrations (0.001-0.1 µg-L-1), increasing photosynthetic activity. The results obtained by Tan et al. (2018) demonstrated that low-level antibiotics exert ecological impacts via interference in aggregation, since this is important to cyanobacterial biofilm formation and consequently to the development of blooms. However, both publications emphasize that a correlation between antibiotic residues in water and the outbreak of algal blooms has not been reported. According to Roose-Amsaleg and Laverman (2015), at least in vitro, sub-inhibitory effects of antibiotics on bacterial physiology can cause mutagenesis, virulence, biofilm formation, and horizontal gene transfer recombination.
Thus, how cyanobacteria behave in the presence of low-level antibiotics can exert both biogeochemical and environmental impacts. Understanding the antibiotic-interfered aggregation process not only helps with understanding of the cyanobacteria-mediated carbon cycle but is also of great importance to predict, evaluate, and control algal blooms (Tan et al., 2018).
Cyanobacterial blooms and their main causes
Cyanobacterial blooms are defined as visible discolorations in water that are caused (predominantly) by cyanobacteria, Gram-negative prokaryotic microorganisms that were originally referred to as the 'blue-green algae. These microorganisms are very closely related to bacteria in terms of cellular structure, with no defined nucleus or membrane-bound organelles present, and constitute oxygen-producing bacteria that use sunlight as an energy source to convert carbon dioxide (CO2) into biomass (Percival and Williams, 2014; Huisman et al., 2018).
Algae and cyanobacteria are a vital part of the food chain in aquatic ecosystems, and even small changes in the algal and cyanobacterial populations could affect the balance of the whole ecosystem (Válitalo et al., 2017). Anthropogenic activities, such as urban, agricultural, and industrial development, are the main source of nutrient over-enrichment of water, the most well-known cause of cyanobacterial blooms (Paerl and Huisman, 2008). Once a cyanobacterial bloom is established, it may persist for months, even after nutrients (N and P) are reduced (Paerl and Otten, 2013).
This type of bloom is recurrently reported in some of the world's largest freshwater ecosystems, such as Lake Victoria (Africa), Lake Erie (USA) and Lake Taihu (China) (Paerl and Otten, 2013). According to Olokotum et al. (2020), the main causes of cyanobacterial blooms in Lake Victoria are due to an increase in human population density, resulting in an increase in food, housing, and product demands (such as agricultural, urban, and industrial activities, respectively), that cause an increase in the release of nutrients to the environment, resulting in eutrophic water bodies.
Michalak et al. (2013), writing in relation to Lake Erie, which experienced one of its largest algal blooms in 2011 due to record-breaking nutrient loads, ascribe cyanobacterial blooms to longterm agricultural activities and to meteorological conditions, such as warm and rainy seasons. Qin et al. (2019), in relation to Lake Taihu which constantly suffers from cyanobacterial blooms, also attribute blooms to lake eutrophication, which generated a commitment from politicians to rehabilitate the lake, accompanied by an investment which has to date amounted to about 14 billion USD. Even so, the lake continues to suffer from cyanobacterial blooms and cyanotoxin release.
According to Carmichael (2001), the main factors influencing formation and toxicity of harmful cyanobacterial blooms are: (i) genetic (distinct toxin- and non-toxin-producing strain); (ii) growth (measured by chlorophyll a, good growth led to optimum toxin production), (iii) ratio of toxin and non-toxin producers, and (iv) bioaccumulation.
Some of the most common toxin-producing cyanobacteria include the N2-fixing genera: Anabaena, Aphanizomenon, Cylindrospermopsis, Lyngbya, Nodularia, Oscillatoria, and Trichodesmium; and the non-N2 fixers: Microcystis and Planktothrix (Paerl and Otten, 2013). Microcystins are the most prevalent class of cyanotoxins and the most frequently studied. The hepatotoxin microcystin originates from several genera of cyanobacteria: Microcystis, Anabaena, Planktothrix, Nostoc, and Anabaenopsis (Westrick et al., 2010).
Release of toxins from cyanobacteria mainly occurs during algal death and cell lysis (Merel et al., 2013; Pham et al., 2020).
According to Liyanage et al. (2016), although algaecides are used to remove cyanobacterial blooms, they are also responsible for cell lysis and the consequent release of toxins. In addition to toxicity, these blooms can cause taste and odour episodes in drinking and recreational water (Carmichael, 2001).
Currently, the presence of cyanobacteria has been reported in Guandu River, and the main water treatment plant (WTP) in the state of Rio de Janeiro, Brazil. At the beginning of 2020 and 2021, the State of Rio de Janeiro faced a major water supply problem due to the presence of an earthy o dour and taste in drinking water, from the presence of GEO and 2-MIB. The presence of these odorous compounds in water bodies associated with cyanobacterial blooms was also recently reported by Asquith et al. (2018); Lee et al. (2020) and Pham et al. (2020), drawing a lot of attention to this association.
Odorous compound release and its main causes
Geosmin (GEO) and 2-methylisoborneol (2-MIB) are the two major volatile organic compounds that contribute to the occurrence of an earthy and musty odour in water, which has been known to decrease its quality, especially when it comes to drinking water (Izaguirre et al., 1982; Lee et al., 2020). Human's sense of smell is sensitive GEO and 2-MIB at levels below 10 ng-L-1 (Suffet et al., 1996). Both are terpenoids and are synthesized from linear isoprene diphosphate precursors with various chain lengths (Pham et al., 2020). They have also been reported to be produced (synthesized and secreted) as secondary metabolites by different types of cyanobacteria (Izaguirre et al., 1982; Carmichael, 2001; Pham et al., 2020), but other studies have shown actinomycetes, proteobacteria, myxobacteria and some fungi to be geosmin producers (Zaitlin and Watson, 2006; John et al., 2018).
GEO and 2-MIB are not considered as health hazards for humans, as it has been shown that environmentally relevant concentrations of both compounds (e.g., ng-µg-L-1) present no cytotoxicity or genotoxicity. However, their presence, even in low concentrations (below 1 µg-L-1 in surface waters and considerably lower in treated drinking water) makes water unacceptable for consumption, which is the main problem they cause for water supplies (Chorus and Welker, 2021).
No official regulatory boundaries were found for the presence of these compounds in water. According to Lee et al. (2020) and Kim and Park (2021), although there is currently no regulation for these two compounds in the Republic of Korea, the Korean government has set 20 ng-L-1 as a maximum allowable limit (MAL) or a maximum contaminant level goal (MCLG) for geosmin and 2-MIB. A preliminary study by Webber et al. (2014) in Australia indicated that drinking water containing greater than 10 ng-L-1 GEO or 2-MIB would result in a reduction in acceptance. Thus, it is believed that the range of 10 to 20 ng-L-1 should be adopted as a parameter of water quality, regarding the presence of GEO and 2-MIB in aqueous medium.
Some researchers have aimed to study the release of these odorous compounds during harmful blooms. According to Pham et al. (2020), GEO and 2-MIB often occurred mainly during the rainy season, which is associated with a high level of nutrients, and when cyanobacterial biomass was high, due to heavy blooms of Microcystis aeruginosa. Lee et al. (2020) observed that temperature was the main parameter affecting cyanobacterial growth, and that the number of cyanobacteria species was positively correlated with geosmin concentration. Asquith et al. (2018), in turn, observed in their study that cyanobacteria are not the unique species responsible for GEO and 2-MIB release, since Streptomyces has also been shown to be responsible for taste and odour episodes in drinking water supplies, especially during rainfall events followed by dry periods.
Alghanmi et al. (2018), when carrying out a study on the effect of light intensity and temperature on the production of GEO and 2-MIB by two cyanobacterial species (Phormidium retzii and Microcoleus vaginatus), observed that odorous compounds are retained in the intracellular fraction in the lag phase, are partially released to the medium in the exponential phase, and are released at high levels to the medium in the stationary and death phases.
As taste and odour compounds can be sensed at very low concentrations, they can serve as an early warning for further investigations regarding the presence of cyanobacteria and, among them, possible cyanotoxin producers (Chorus and Welker, 2021).
RESULTS AND DISCUSSION
Antibiotics toxicity to cyanobacteria
The bibliometric analysis map created in the VOSviewer program for the first search (keywords 'antibiotics', 'toxicity' and 'cyanobacteria') is shown in Fig. 1; 55 results were found for this search. It is possible to observe the formation of four clusters, observable as four different colours (red, green, blue, and yellow). The list of words in each cluster is shown in Table 2.
Cluster 1 (red) is commonly related to the presence of antibiotics and pharmaceuticals in water bodies, as well as their toxicity to algae and cyanobacterial species. 'Antibiotics' and 'cyanobacteria' were the keywords with the highest co-occurrence, with both achieving a score of 26. Although not prominently shown on the map, it is worth mentioning that two antibiotics in this cluster, ciprofloxacin and trimethoprim, were the most frequently detected in the aquatic environment.
Cluster 2 (green) mainly refers to the toxicity of antibiotics to cyanobacterial species, especially to the species Microcystis aeruginosa. The terms 'toxicity' and 'growth' showed the highest co-occurrence values with scores of31 and 15, respectively. The presence of the antibiotic erythromycin in this cluster is noteworthy.
Cluster 3 (blue) has as its main keywords the term 'pharmaceuticals', with 16 occurrences, and 'personal care products' with 10 occurrences, referring, therefore, to the main contaminants identified in water bodies. It is interesting to highlight the presence of the word 'China' in this cluster, since this country is reported in the literature as being one of the biggest consumers of antibiotics in the world (Qiao et al., 2018).
Cluster 4 (yellow), in turn, focuses on the fate of these contaminants in nature, pointing to the risk they can cause to aquatic species. The most frequent words were 'waste-water' and 'antibacterial agents' with scores of 14 and 6, respectively.
Thus, in general, the bibliometric survey gave results in accordance with what is reported in the literature presented, since the most frequent terms in each cluster were also those discussed in the review in the previous section, highlighting the presence of antibiotics in the aquatic environment and its toxicity to cyanobacterial species.
Cyanobacterial blooms and their main causes
The network visualization map for the second search (keywords 'cyanobacteria', 'blooms' and 'causes') is shown in Fig. 2; 128 results were found for this search. As for the first search, it is possible to observe the formation of four clusters. Table 3 presents the list of words in each cluster.
It is possible to observe in Cluster 1 (red) that the most frequent words are 'blooms' and 'growth', both with a score of 22. This is because blooms, especially cyanobacterial blooms, are related to the exacerbated growth of microorganisms, as pointed out in the literature review. The terms 'climate change' and 'light' constitute, in this cluster, the main causes of blooms. Thus, this cluster refers specifically to the dynamics of cyanobacterial blooms. It is worth mentioning the presence of the term 'Lake Taihu', one of the main lakes in China that constantly suffers from cyanobacterial blooms.
Cluster 2 (green), in turn, refers to the toxicity that cyanobacterial blooms exert on the aquatic environment. The words 'climate-change', 'harmful algal blooms' and 'Microcystis-aeruginosa' stand out, with scores of 25, 19 and 13, respectively. Again, the climate change that the world has been facing in recent years is pointed out as the main cause of these blooms, mainly indicated by the presence of the term 'temperature' in this cluster.
The third cluster (blue) relates to the identification of cyanotoxins in fresh water, especially microcystins produced by Microcystis aeruginosa. The most frequent words were 'cyanobacteria' with a score of 53, and 'fresh-water' with a score of 17. Therefore, the release of toxic products by cyanobacteria is directly related to cyanobacterial blooms.
The last cluster (yellow) refers to the best-known cause of cyanobacterial blooms: eutrophication of water bodies. Therefore, the most frequent words were 'eutrophication', 'phytoplankton', 'nitrogen' and 'phosphorus, with scores of 32, 21, 18 and 16, respectively.
Again, the bibliometric analysis agrees with what was pointed out previously, except for the mention of rainy seasons as one of the causes of cyanobacterial blooms.
Odorous compound release and its main causes
Figure 3 presents the network visualization map for the third and last search in the WOS database (keywords 'geosmin', '2-methylisoborneol' and 'cyanobacteria'); 162 results were found for this search. Table 4 presents the list of words in each of the four clusters.
The most frequent words in Cluster 1 (red) are 'cyanobacteria, 'taste', 'drinking-water', 'MIB', 'removal' and 'odor compounds', with scores of 107, 58, 49, 36, 27 and 21, respectively. It is observed that this cluster refers, therefore, to the presence of odorous compounds in water, especially water for human consumption. Furthermore, it turns to the discussion of odorous compound removal methods, having as alternatives the use of adsorptive processes through activated carbon and oxidative processes such as ozonation.
In Cluster 2 (green), the terms 'biosynthesis', '2-MIB, 'temperature, 'lake' and 'growth, with scores of 27, 19, 16, 16 and 15, respectively, stand out. In addition, there are several terms already seen in previous analyses, such as the main causes of cyanobacterial blooms ('light', 'eutrophication', 'temperature'), among others.
Cluster 3 (blue) refers to the impact of the presence of odoriferous compounds on aquatic species, such as fish, for example. The most frequent words are 'geosmin' with a score of 127, '2-methylisoborneol' with a score of 108, 'water' with a score of 20 and 'off-flavor' with a score of 16. Again, we highlight the presence of the term 'Lake Taihu, as noted for the previous search.
Cluster 4 (yellow), in turn, refers to quantification and identification of the gene expression of odoriferous compounds, especially by the most recent method mentioned in the literature, qPCR. The most frequent words were 'identification' with a score of 43, 'odor' with a score of 31 and 'reservoir' with a score of 17.
In this way, the map reveals exactly what was noted in the literature review, with the addition of the most common processes used for the removal and remediation of these types of compounds in water.
Graphical summary of results
Using the data presented by the network visualization maps generated by the VOSviewer software, a graphical analysis (Fig. 4) was carried out to demonstrate the keywords identified to be in common across the three analyses. Based on the data for the three main points of interest of this research study ('antibiotics', 'cyanobacterial blooms' and 'odoriferous compounds'), it is possible to raise some questions about the relationships between them.
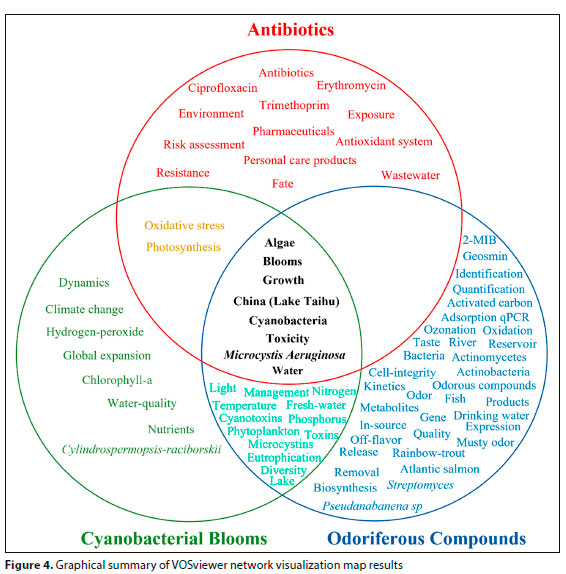
It can be observed that, in the literature, there is no relationship seen between antibiotics and odoriferous compounds, as already mentioned in the introduction to this study. This is because there is no direct and explicit correlation in the literature between the presence of antibiotics in water bodies and the release of compounds such as GEO and 2-MIB. However, based on the keyword analysis, there is a relationship, albeit small, between antibiotics and cyanobacterial blooms. Highlighted words are 'oxidative stress' and 'photosynthesis', from the metabolic stress and inhibition that this class of drugs exerts on the cyanobacterial community.
It is observed, however, that the greatest relationship between the two points of interest is found between cyanobacterial blooms and the release of odoriferous compounds, presenting terms related to the main causes of cyanobacterial blooms ('light, 'temperature, 'eutrophication', 'nitrogen', 'phosphorus'), as well as the release of cyanotoxins such as microcystins in freshwater and lakes. Therefore, as explained in the literature review, cyanobacterial blooms are the main organisms responsible for the release of odoriferous compounds in water bodies.
The most interesting result, however, was the one that relates the common terms of the three major points of interest analysed. The words 'algae', 'blooms, 'growth, 'China/Lake Taihu', 'cyanobacteria, 'toxicity', 'Microcystis aeruginosa' and 'water' appeared in the three network visualization maps. This supports the proposal that the presence of antibiotics in water bodies may also be one of the causes of the release of odoriferous compounds in the aquatic environment.
As seen in the graph, this is not a direct relationship, nor has it been reported in the literature as such. Therefore, the importance of this work is in highlighting the need for further research in the form of experimental investigation of the relationship between the presence of antibiotics and odoriferous compound release.
Proposed relationship between antibiotics, cyanobacterial blooms and odorous compound release
Based on the bibliometric analyses and all the articles consulted and reported on in the literature review, it is believed that there is a relationship between the presence of antibiotics in water bodies and the occurrence of cyanobacterial blooms capable of causing the release of odorous compounds in aqueous media, and causing problems in terms of the water quality consumed by the population. Some ofthe most important points of this relationship will be highlighted here, such as some associations for which cases have already been reported in the literature.
According to Liu et al. (2012), antibiotic contaminants in the aquatic environment are likely to be an emerging environmental factor regulating the growth of cyanobacteria and the production and release of microcystins (cyanotoxins) by cyanobacteria. Liu et al. (2014) affirm that coexisting antibiotic contaminants have the potential to regulate cyanobacterial blooms. Tan et al. (2018) discovered that low-level antibiotics induce the aggregation of cyanobacteria, and these increased aggregations would further influence biofilm growth and, consequently, algal blooms.
As previously mentioned, cyanobacterial blooms are currently repeatedly reported for the world's largest freshwater ecosystems, such as Lake Victoria (Africa), Lake Erie (USA) and Lake Taihu (China) (Paerl and Otten, 2013).
Data obtained by WHO (2018), indicate that the main causes of death from infectious diseases in African in the year 2015 were lower respiratory infections, HIV/AIDS, diarrhoeal diseases, malaria and tuberculosis. According to Dalahmeh et al. (2019), in Uganda, Kenya and other African countries, sulfamethoxazole combined with trimethoprim is used to treat several bacterial infections, including being used for the treatment for children of HIV-infected mothers. Besides that, Uganda is an endemic malaria region, explaining the high consumption of pharmaceuticals and, consequently, the high disposal. Thus, their study reported a significant concentration of trimethoprim and sulfamethoxazole from Bugolobi Wastewater Treatment Plant to Nakivubo Channel.
Nantaba et al. (2019) reported the presence of 24 pharmaceuticals, the majority being classified as antibiotics, near Lake Victoria. The most predominant antibiotics were sulfamethoxazole, trimethoprim, and oxytetracycline, a result that corroborates what was reported by Dalahmeh et al. (2019). According to Nantaba et al. (2019) pharmaceutical industry wastewater discharge, wastewater effluents, municipal waste and fish farms were the probable sources of these contaminants in the studied water body.
The Center for Disease Control and Prevention (CDC, 2019) reported in their update on USA antibiotic use that too many antibiotics are prescribed unnecessarily in the country. They estimate that about 47 million doses of this class of pharmaceutical are prescribed in USA doctors' offices and emergency departments each year for infections that do not need antibiotics, and that this number corresponds to 30% of all antibiotics prescribed. Thus, it is understood that the CDC has knowledge of the use and improper prescription of this type of medication and promote guidance campaigns aiming at correct use and prescription of these drugs.
No studies were found in the literature that directly reported the presence of antibiotics in Lake Erie. However, Skwor et al. (2014) reported the prevalence of tetracycline and ciprofloxacin resistance among potentially pathogenic Aeromonas isolates from the eastern basin of Lake Erie, indicating the supposed presence of these two antibiotics in the lake.
China is one of the world's largest producers and consumers of antibiotics, widely used for disease treatment in humans and livestock, and as prophylaxis and growth promoters for the latter (Qiao et al., 2018). According to Yin et al. (2013), approximately 50% ofhospital outpatients in China are reported to use antibiotics. Of these outpatients, 74.0% were prescribed one antibiotic, and 25.3% were prescribed two or more antibiotics.
In agreement with Yin et al. (2013), although an international standard percentage use of this class of drug has not been empirically established, WHO (2006) has recommended that the proportion of antibiotic use should not be >30% of prescribed medicines. However, the percentage use of antibiotics in China (50.3%) is much higher than this recommended level and higher than in many other countries.
According to Yin et al. (2013), high percentages of antibiotic-resistant bacteria were detected in Lake Taihu, due to the presence of streptomycin, ampicillin, tetracycline, and chloramphenicol in water. They believe that the increase in bacterial resistance is associated with the high level of pollution of the lake, since it is located close to an urban area and suffers from intense effluent discharges.
Xu et al. (2014) studied the occurrence of 15 antibiotics, such as sulphonamides, fluoroquinolones, macrolides, tetracyclines and trimethoprim in sediment, overlying water, and pore water matrices in Lake Taihu. Analysis of the composition of the studied antibiotics indicated that human-derived and animal-derived drugs contributed significantly to the total contamination of the lake.
It is worth mentioning that the studies cited above have focused on the problem of antibiotic presence in water bodies, and their association to urbanization, agricultural activities, and industrial development (anthropogenic activities), which is also the main source of cyanobacterial blooms, which usually cause cyanotoxin and odorous compound release in aqueous environments.
Lee et al. (2020) observed that cyanobacteria and geosmin increased and decreased in a similar temporal pattern at the water reservoirs studied, indicating that cyanobacteria likely were the dominant producer of geosmin. The same behaviour was observed by Pham et al. (2020), where GEO and 2-MIB often occurred when cyanobacterial biomass was high.
Thus, it is assumed that the problems with taste and odour compounds faced by many populations is due to antibiotics' toxicity to cyanobacteria, which are associated with eutrophic, polluted, warm and rainy (due to tropical weather) aquatic environments and produced GEO and 2-MIB. No study was found in the literature that has proposed this relationship. Thus, batch experiments with all possible variables should be carried out to confirm and investigate the relationship here proposed.
CONCLUSION
Based on the literature review presented and bibliometric analyses by means of network visualization maps plotted by VOSviewer software, it was observed that:
• Several studies point to the toxicity that antibiotics present to cyanobacterial species, by means of oxidative stress, photosynthesis inhibition or regulation of cyanobacterial growth.
• The main conditions of cyanobacterial bloom occurrence are a eutrophic environment with high temperatures and during rainy seasons. In parallel, the number of articles that discuss recurrent cyanobacterial blooms is increasing and, coincidentally or not, other articles show the constant presence of antibiotics in these locations.
• Odorous compound release is directly related to cyanobacterial blooms, especially when they suffer cell lysis or die.
Therefore, it is believed that one of the causes of cyanobacterial blooms and consequently odorous compounds release, to date never mentioned in the literature, is the presence and recalcitrance of antibiotics in water bodies, responsible for toxicity to cyanobacterial species, consequently promoting the release of odoriferous compounds.
AUTHOR CONTRIBUTIONS
BL Novo: writing - original draft, writing - reviewing and editing, investigation, formal analysis, conceptualization, visualization. Fernanda ANG Silva: supervision, writing - reviewing and editing, resources, formal analysis, conceptualization. Luiz C Bertolino: supervision, writing - reviewing and editing, resources, formal analysis, conceptualization. L Yokoyama: supervision, writing - reviewing and editing, resources, formal analysis, conceptualization.
ACKNOWLEDGEMENTS
Thanks go to CNPq (140198/2021-0) and FAPERJ (26/211.688/2021) for their financial aid and CAPES for its scientific support.
ORCIDS
Bruna de Lemos Novo: https://orcid.org/0000-0002-5889-8082
Fernanda Arruda Nogueira Gomes da Silva: https://orcid.org/0000000183989974
Luiz Carlos Bertolino: https://orcid.org/0000-0002-0908-180X
Lidia Yokoyama: https://orcid.org/0000000223174690
REFERENCES
ALGHANMI HA, ALKAM FM and AL-TAEE MM (2018) Effect of light and temperature on new cyanobacteria producers for geosmin and 2-methylisoborneol. J. Appl. Phycol. 30 319-328. https://doi.org/10.1007/s10811-017-1233-0 [ Links ]
ANDREOZZI R, CAPRIO V, CINIGLIA C, DE CHAMPDORÉ M, LO GIUDICE R, MAROTTA R and ZUCCATO E (2004) Antibiotics in the environment: occurrence in Italian STPs, fate, and preliminary assessment on algal toxicity of amoxicillin. Environ. Sci. Technol. 38 (24) 6832-6838. https://doi.org/10.1021/es049509a [ Links ]
ASQUITH E, EVANS C, DUNSTAN RH, GEARY P and COLE B (2018) Distribution, abundance and activity of geosmin and 2-methylisoborneol-producing streptomyces in drinking water reservoirs. Water Res. 145 30-38. https://doi.org/10.1016/j.watres.2018.08.014 [ Links ]
BENGTSSON-PALME J and LARSSON DGJ (2016) Concentrations of antibiotics predicted to select for resistant bacteria: Proposed limits for environmental regulation. Environ. Int. 86 140-149. https://doi.org/10.1016/j.envint.2015.10.015 [ Links ]
BUENO MJM, GOMEZ MJ, HERRERA S, HERNANDO MD, AGÜERA A and FERNÁNDEZ-ALBA AR (2012) Occurrence and persistence of organic emerging contaminants and priority pollutants in five sewage treatment plants of Spain: Two years pilot survey monitoring. Environ. Pollut. 164 267-273. https://doi.org/10.1016/j.envpol.2012.01.038 [ Links ]
CARMICHAEL WW (2001) Health effects of toxin-producing cyanobacteria: "The CyanoHABs". Hum. Ecol. Risk Assess. 7 (5) 1393-1407. https://doi.org/10.1080/20018091095087 [ Links ]
CDC (Center for Disease Control, United States) (2019) Antibiotic use in the United States, 2018 update: progress and opportunities. US Department of Health and Human Services, CDC, Atlanta, GA. [ Links ]
CHORUS I and WELKER M (2021) Toxic Cyanobacteria in Water (2nd edn). CRC Press, Boca Raton (FL). On behalf of the World Health Organization, Geneva, CH. 859 pp. [ Links ]
CHOW LKM, GHALY TM and GILLINGS MR (2021) A survey of sub-inhibitory concentrations of antibiotics in the environment. J. Environ. Sci. 99 21-27. https://doi.org/10.1016Zj.jes.2020.05.030 [ Links ]
DALAHMEH S, BJÖRNBERG E, ELENSTRÖM A-K, NIWAGABA CB and KOMAKECH AJ (2019) Pharmaceutical pollution of water resources in Nakivubo wetlands and Lake Victoria, Kampala, Uganda. Sci. Total Environ. 710 136347. https://doi.org/10.1016/j.scitotenv.2019.136347 [ Links ]
DIAS E, OLIVEIRA M, JONES-DIAS D, VASCONCELOS V, FERREIRA E, MANAGEIRO V and CANIÇA M (2015) Assessing the antibiotic susceptibility of freshwater cyanobacteria spp. Front. Microbiol. 6. https://doi.org/10.3389/fmicb.2015.00799 [ Links ]
DING C and HE J (2010) Effect of antibiotics in the environment on microbial populations. Applied Microbiol. Biotechnol. 87 (3) 925941. https://doi.org/10.1007/s00253-010-2649-5 [ Links ]
GONZÁLEZ-PLEITER M, LEGANÉS F and FERNÁNDEZ-PINAS F (2017) Intracellular free Ca2+ signals antibiotic exposure in cyanobacteria. RSC Adv. 7 (56) 35385-35393. https://doi.org/10.1039/c7ra03001k [ Links ]
GUO J, SELBY K and BOXALL ABA (2016) Comparing the sensitivity of chlorophytes, cyanobacteria, and diatoms to major-use antibiotics. Environ. Toxicol. Chem. 35 (10) 2587-2596. https://doi.org/10.1002/etc.3430 [ Links ]
HALLING-S0RENSEN B (2000) Algal toxicity of antibacterial agents used in intensive farming. Chemosphere 40 731-739. https://doi.org/10.1016/S0045-6535(99)00445-2 [ Links ]
HIRSCH R, TERNES T, HABERER K and KRATZ KL (1999) Occurrence of antibiotics in the aquatic environment. Sci. Total Environ. 225 109-118. https://doi.org/10.1016/S0048-9697(98)00337-4 [ Links ]
HOLTEN-LÜTZH0FT HC, HALLING-S0RENSEN B and J0RGENSEN SE (1999) Algal toxicity of antibacterial agents applied in Danish fish farming. Arch. Environ. Contamin. Toxicol. 36 1-6. https://doi.org/10.1007/s002449900435 [ Links ]
HUISMAN J, CODD GA, PAERL HW, IBELINGS BW, VERSPAGEN JMH and VISSER PM (2018) Cyanobacterial blooms. Nat. Rev. Microbiol. 16 471-483. https://doi.org/10.1038/s41579-018-0040-1 [ Links ]
IZAGUIRRE G, HWANG CJ, KRASNER SW and MCGUIRE MJ (1982) Geosmin and 2-methylisoborneol from cyanobacteria in three water supply systems. Appl. Environ. Microbiol. 43 (3) 708-714. https://doi.org/10.1128/AEM.43.3.708-714.1982 [ Links ]
JOHN N, KOEHLER AV, ANSELL BRE, BAKER L, CROSBIE ND and JEX AR (2018) An improved method for PCR-based detection and routine monitoring of geosmin-producing cyanobacterial blooms. Water Res. 136 34-40. https://doi.org/10.1016/j.watres.2018.02.041 [ Links ]
KIM KT and PARK Y-G (2021) Geosmin and 2-MIB removal by full-scale drinking water treatment processes in the Republic of Korea. Water 13 (5) 628. https://doi.org/10.3390/w13050628 [ Links ]
KOVALAKOVA P, CIZMAS L, MCDONALD TJ, MARSALEK B, FENG M and SHARMA VK (2020) Occurrence and toxicity of antibiotics in the aquatic environment: A review. Chemosphere 251 126351. https://doi.org/10.1016/jxhemosphere.2020.126351 [ Links ]
KÜMMERER K (2009) Antibiotics in the aquatic environment - A review - Part I. Chemosphere 75 (4) 417-434. https://doi.org/10.1016/j.chemosphere.2008.11.086 [ Links ]
LEE JE, YOUN S-J, BYEON M and YU S (2020) Occurrence of cyanobacteria, actinomycetes, and geosmin in drinking water reservoir in Korea: a case study from an algal bloom in 2012. Water Supply 20 (5) 1862-1870. https://doi.org/10.2166/ws.2020.102 [ Links ]
LIU Y, CHEN X, ZHANG J and GAO B (2014) Hormesis effects of amoxicillin on growth and cellular biosynthesis of Microcystis aeruginosa at different nitrogen levels. Microb. Ecol. 69 (3) 608-617. https://doi.org/10.1007/s00248-014-0528-9 [ Links ]
LIU Y, GAO B, YUE Q, GUAN Y, WANG Y and HUANG L (2012) Influences of two antibiotic contaminants on the production, release and toxicity of microcystins. Ecotoxicol. Environ. Saf. 77 79-87. https://doi.org/10.1016/j.ecoenv.2011.10.027 [ Links ]
LIYANAGE HM, MAGANA ARACHCHI DN, ABEYSEKARA T and GUNERATNE L (2016) Toxicology of freshwater cyanobacteria. J. Environ. Sci. Health C 34 (3) 137-168. https://doi.org/10.1080/10590501.2016.1193923 [ Links ]
MEREL S, WALKER D, CHICANA R, SNYDER S, BAURÈS E and THOMAS O (2013) State of knowledge and concerns on cyanobacterial blooms and cyanotoxins. Environ. Int. 59 303-327. https://doi.org/10.1016/j.envint.2013.06.013 [ Links ]
MICHALAK AM, ANDERSON EJ, BELETSKY D, BOLAND S, BOSCH NS, BRIDGEMAN TB, CHAFFIN JD, CHO K, CONFESOR R, DALOGLU I and co-authors (2013) Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl Acad. Sci. 110 (16) 6448-6452. https://doi.org/10.1073/pnas.1216006110 [ Links ]
MOG M, NGASOTTER S, TESIA S, WAIKHOM D, PANDA S, SHARMA S and VARSHNEY S (2020) Problems of antibiotic resistance associated with oxytetracycline use in aquaculture: A review. J. Entomol. Zool. Stud. 8 1075-1082 [ Links ]
NANTABA F, WASSWA J, KYLIN H, PALM W-U, BOUWMAN H and KÜMMERER K (2019) Occurrence, distribution, and ecotoxicological risk assessment of selected pharmaceutical compounds in water from Lake Victoria, Uganda. Chemosphere 239 124642. https://doi.org/10.1016/jxhemosphere.2019.124642 [ Links ]
OLOKOTUM M, MITROI V, TROUSSELLIER M, SEMYALO R, BERNARD C, MONTUELLE B, OKELLO W, QUIBLIER C and HUMBERT J-Fb (2020) A review of the socioecological causes and consequences of cyanobacterial blooms in Lake Victoria. Harmful Algae 96 101829. https://doi.org/10.1016/j.hal.2020.101829 [ Links ]
PAERL HW and HUISMAN J (2008) Blooms like it hot. Science 320 57-58. https://doi.org/10.1126/science.1155398 [ Links ]
PAERL HW, HALL NS and CALANDRINO ES (2011) Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ. 409 (10) 1739-1745. https://doi.org/10.1016/j.scitotenv.2011.02.001 [ Links ]
PAERL HW and OTTEN TG (2013) Harmful cyanobacterial blooms: causes, consequences and controls. Microb. Ecol. 65 995-1010. https://doi.org/10.1007/s00248-012-0159-y [ Links ]
PAN X, DENG C, ZHANG D, WANG J, MU G and CHEN Y (2008) Toxic effects of amoxicillin on the photosystem II of Synechocystis sp. characterized by a variety of in vivo chlorophyll fluorescence tests. Aquat. Toxicol. 89 (4) 207-213. https://doi.org/10.1016/j.aquatox.2008.06.018 [ Links ]
PERCIVAL SL and WILLIAMS DW (2014) Cyanobacteria. In: Percival SL, Yates MV, Williams DW, Chalmers RM and Gray NF (eds) Microbiology of Waterborne Diseases (Second Edition), Academic Press. https://doi.org/10.1016/b978-0-12-415846-7.00005-6 [ Links ]
PHAM T-L, BUI MH, DRISCOLL M, SHIMIZU K and MOTOO U (2020) First report of geosmin and 2-methylisoborneol (2-MIB) in Dolichospermum and Oscillatoria from Vietnam. Limnology 1 43-56. https://doi.org/10.1007/s10201-020-00630-2 [ Links ]
QIAO M, YING G-G, SINGER AC and ZHU Y-G (2018) Review of antibiotic resistance in China and its environment. Environ. Int. 110 160-172. https://doi.org/10.1016/j.envint.2017.10.016 [ Links ]
QIN B, PAERL HW, BROOKES JD, LIU J, JEPPESEN E, ZHU G, ZHANG Y, XU H, SHI K and DENG J (2019) Why Lake Taihu continues to be plagued with cyanobacterial blooms through 10 years (2007-2017) efforts. Sci. Bull. 64 (6) 354-356. https://doi.org/10.1016/j.scib.2019.02.008 [ Links ]
RICO A, ZHAO W, GILLISSEN F, LÜRLING M and VAN DEN BRINK PJ (2018) Effects of temperature, genetic variation and species competition on the sensitivity of algae populations to the antibiotic enrofloxacin. Ecotoxicol. Environ. Saf. 148 228-236. https://doi.org/10.1016/j.ecoenv.2017.10.010 [ Links ]
ROOSE-AMSALEG C and LAVERMAN AM (2015) Do antibiotics have environmental side-effects? Impact of synthetic antibiotics on biogeochemical processes. Environ. Sci. Pollut. Res. 23 (5) 4000-4012. https://doi.org/10.1007/s11356-015-4943-3 [ Links ]
SKWOR T, SHINKO J, AUGUSTYNIAK A, GEE C and ANDRASO G (2013) Aeromonas hydrophila and Aeromonas veronii predominate among potentially pathogenic ciprofloxacin- and tetracycline-resistant Aeromonas isolates from Lake Erie. Appl. Environ. Microbiol. 80 (3) 841-848. https://doi.org/10.1128/aem.03645-13 [ Links ]
SUFFET IH, CORADO A, CHOU D, MCGUIRE MJ and BUTTERWORTH S (1996) AWWA taste and odor survey. J. Am. Water Works Assoc. 88 168-180. https://doi.org/10.1002/j.1551-8833.1996.tb06542.x [ Links ]
TAN L-R, XIA P-F, ZENG RJ, LI Q, SUN X-F and WANG S-G (2018) Low-level concentrations of aminoglycoside antibiotics induce the aggregation of cyanobacteria. Environ. Sci. Pollut. Res. 25 (17) 17128-17136. https://doi.org/10.1007/s11356-018-1894-5 [ Links ]
VÀLITALO P, KRUGLOVA A, MIKOLA A and VAHALA R (2017) Toxicological impacts of antibiotics on aquatic micro-organisms: A mini-review. Int. J. Hyg. Environ. Health 220 (3) 558-569. https://doi.org/10.1016/j.ijheh.2017.02.003 [ Links ]
VAN DER GRINTEN E, PIKKEMAAT MG, VAN DEN BRANDHOF E-J, STROOMBERG, GJ and KRAAK MHS (2010) Comparing the sensitivity of algal, cyanobacterial and bacterial bioassays to different groups of antibiotics. Chemosphere 80 1-6. https://doi.org/10.1016/j.chemosphere.2010.04.011 [ Links ]
VAN ECK NJ and WALTMAN L (2010) Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84 523-538. https://doi.org/10.1007/s11192-009-0146-3 [ Links ]
VAN ECK NJ and WALTMAN L (2022) VOSviewer Manual. Manual for VOSviewer version 1.6.18. [ Links ]
WAN J, GUO P, PENG X and WEN K (2015) Effect of erythromycin exposure on the growth, antioxidant system and photosynthesis of Microcystis flos-aquae. J. Hazardous Mater. 283 778-786. https://doi.org/10.1016/j.jhazmat.2014.10.026 [ Links ]
WEBBER MA, ATHERTON P and NEWCOMBE G (2014) Water. J. Aus. Water Assoc. 41 (4) 48-52. [ Links ]
WESTRICK JA, SZLAG DC, SOUTHWELL BJ and SINCLAIR J (2010) A review of cyanobacteria and cyanotoxins removal/ inactivation in drinking water treatment. Anal. Bioanal. Chem. 397 (5) 1705-1714. https://doi.org/10.1007/s00216-010-3709-5 [ Links ]
WHO (World Health Organisation) (2006) Using indicators to measure country pharmaceutical situations. Fact Book on WHO Level I and Level II monitoring indicators. WHO, Geneva. URL: https://apps.who.int/iris/bitstream/handle/10665/354554/WHO-TCM-2006.2-eng.pdf [ Links ]
WHO (World Health Organisation) (2018) WHO report on surveillance of antibiotic consumption: 2016-2018 early implementation. WHO, Geneva. ISBN: 978-92-4-151488-0. [ Links ]
XU J, ZHANG Y, ZHOU C, GUO C, WANG D, DU P, LUO Y, WAN J and MENG W (2014) Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China. Sci. Total Environ. 497-498 267-273. https://doi.org/10.1016/j.scitotenv.2014.07.114 [ Links ]
YE J, DU Y, WANG L, QIAN J, CHEN J, WU Q and HU X (2017) Toxin release of cyanobacterium Microcystis aeruginosa after exposure to typical tetracycline antibiotic contaminants. Toxins 9 (2) 53. https://doi.org/10.3390/toxins9020053 [ Links ]
YIN Q, YUE D, PENG Y, LIU, Y and XIAO L (2013) Occurrence and distribution of antibiotic-resistant bacteria and transfer of resistance genes in Lake Taihu. Microbes Environ. 28 (4) 479-486. https://doi.org/10.1264/jsme2.me13098 [ Links ]
YIN X, SONG F, GONG Y, TU X, WANG Y, CAO S, LIU J and LU Z (2013) A systematic review of antibiotic utilization in China. J. Antimicrob. Chemother. 68 (11) 2445-2452. https://doi.org/10.1093/jac/dkt223 [ Links ]
ZAITLIN B and WATSON SB (2006) Actinomycetes in relation to taste and odor in drinking water: Myths, tenets and truths. Water Res. 40 (9) 1741-1753. https://doi.org/10.1016/j.watres.2006.02.024 [ Links ]
ZHANG Q, ZHANG Z, LU T, PEIJNENBURG WJGM, GILLINGS M, YANG X, CHEN J, PENUELAS J, ZHU Y-G, ZHOU N-Y and co-authors (2020) Cyanobacterial blooms contribute to the diversity of antibiotic-resistance genes in aquatic ecosystems. Commun. Biol. 3 737. https://doi.org/10.1038/s42003-020-01468-1 [ Links ]
 Correspondence:
Correspondence:
Bruna de Lemos Novo
Email:brunalemosnovo@eq.ufrj.br
Received: 7 October 2022
Accepted: 6 September 2023














