Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.49 n.3 Pretoria Jul. 2023
http://dx.doi.org/10.17159/wsa/2023.v49.i3.3906
TECHNICAL NOTE
The inability of organic coagulants to purify potable water to its best attainable quality
P Polasek; CJ Wantenaar
P Polasek & Associates, PO Box 3136, Kyalami 1684, South Africa
ABSTRACT
Organic coagulants have gained increasing popularity over the past 30 years because they are considered cheaper and more user-friendly than traditional mineral hydrolysing coagulants. Regrettably, in-depth studies have not yet been conducted on their ability to purify water to a healthy palatable drinking quality which is risk-free for lifetime consumption in terms of the national standard for drinking water quality of various countries worldwide, and does not become a source of secondary pollution in the reticulation system. The objective of this paper is to provide information about the natural organic matter (NOM) removal efficiency attained at different waterworks purifying water with different organic coagulants, and to compare this with that attained using mineral coagulants. The findings presented in this article prove that organic coagulants are not an equivalent replacement for mineral coagulants, as the purified water is of an inferior quality which does not comply with the limits set by national standards of different countries worldwide.
Keywords: health risk, hydrolysing coagulants, NOM removal, organic coagulants, turbidity removal
INTRODUCTION
The main aim of water purification for domestic water supply is to produce a wholesome drinking water which is palatable, healthy and risk-free for lifetime consumption, and which also does not become a source of secondary pollution in the reticulation system. This means that all undesirable, health-risk pollutants must be removed from the water. This must be achieved economically at the maximum attainable efficiency. The water purification processes must be so designed that water is purified to a quality compatible with that determined for domestic use by the national standards of different countries worldwide.
The focus of this paper is on water-soluble cationic polymers, referred to as organic coagulants or polymeric coagulants, and not on anionic and non-ionic polymers, which function differently.
The objective of this paper is to provide decision makers, waterworks designers, supervisors and process controllers with information substantiating the claim that no organic coagulants are capable of purifying water to the quality suitable for domestic supply because they are not capable of efficiently removing natural organic matter (NOM) as well as turbidity from the purified water; NOM is considered to be the most undesirable health-risk pollutant in drinking water. In addition, other detrimental side-effects, i.e. on filtration, have also not been properly investigated or their elimination addressed.
The mineral and organic coagulants are also referred to as aggregation agents. The mineral coagulants are hydrolysing coagulants. Organic coagulants, also termed polymeric coagulants and bio-coagulants, may be of synthetic or natural origin. They are applied neat or as a blend, often with a hydrolysing coagulant. The types of organic coagulants tested in this research were different cation-active water-soluble polymers.
A number of articles have been published on the application of organic coagulants for purification of water and wastewater. Amran et al. (2018) published an article entitled 'The effectiveness of natural coagulant in coagulation process: a review. This article summarises the impact of organic coagulants on water purification, and points out that a number of potential research gaps exist around certain aspects of natural coagulants in water and wastewater treatment. One such gap relates to the connection between increases in organic matter and chlorine use. Use of natural coagulants inevitably increases the amount of organic matter present in the treated water - this will increase microbial activity which then requires higher chlorine use. There has, however, been limited documentation of how these parameters influence one another. In addition, though natural coagulants are considered to be a cheaper option than applying chemical coagulants, an increase in chlorine use could actually increase the cost of water treatment (Amran et al., 2018). This review substantiates the poor efficiency of organic coagulants for the removal of NOM and even shows an increase in NOM in the purified water.
The review by Amran et al. (2018) also proposes that one of the reasons why natural coagulants have gained in popularity over chemical coagulants is that they are perceived to be safer than chemical coagulants. When using a hydrolying coagulant in water treatment, the possibility exists for residual cations to be present in the water post-treatment. Chemical coagulant residues such as alum are considered harmful as aluminium has been suggested as being a causal factor for Alzheimer's disease. The presumption of residual alum is correct only when the reaction conditions under which the purification process takes place are not optimised. When they are optimised there is no Al or Fe coagulant residue left in the purified water or it is well within the limits permitted by the national standards for drinking water quality of different countries worldwide.
Water is not polluted by a single type of impurity but by a number of different impurities of various types. The impurities are characterised by a high degree of dispersion, and are kept suspended by their aggregate and kinetic stability. Impurities include hydrophobic colloids, such as turbidity, and cations of hydrolysing coagulants and hydrophilic colloids, such as NOM of various types. The hydrophobic colloids are mainly surface stabilised, usually by a negative charge (electric double layer). The hydrophilic colloids are stabilised by a hydration layer and their negative charge is caused by ionisation of carboxyl and phenolic functional groups (-COOH and -OH) (Pitter, 1981). In some instances, when both hydrophobic and hydrophilic dispersions are present, a hydrophobic colloid can be surrounded by a hydrophilic colloid thus producing a protective colloid. The protective colloid appears as hydrophobic but behaves as a hydrophilic colloid and is the most difficult to remove.
Organic impurities are composed of a broad spectrum of substances of natural and synthetic origin and with different molecular weights. Organic impurities in surface waters are of natural and anthropogenic origin. The sources of NOM (humic substances and proteins) are soil and sediment extracts, metabolic activities and decaying plants and animals. The sources of anthropogenic organic matter are sewage and industrial wastewaters, effluents and runoff from agriculture, etc. Both types can be present in the form of analytical and colloidal dispersions or in the form of suspensions.
NOM constitutes the most important and the most undesirable group of substances which constitute a serious health risk, as explained by Hocman (1986):
• The most serious organic pollution is caused by humic matters, as these are significant precursors of organohalogens and other chlorine-derivates known to be carcinogens.
• The chemical, biological and hygienic properties of water are affected by NOM, which can have toxicogenic, mutagenic, allergenic and teratogenic effects. Some NOM are not toxic on their own, but alter the taste and odour of water and some may become toxic during purification processes, e.g. chlorination.
• Some NOM (humic matter, amino acids, polysacharides) can form complexes with metals, which then prevents their removal from the water. Products generated by the life and decay of organisms such as actinomycetes and algae, are also an undesirable group of organic matter from a hygienic point of view.
• Failure to remove NOM during the purification process can result in their becoming a source of secondary pollution as they provide a source of carbon which facilitates the development of micro-organisms in the reticulation system.
NOM is most commonly produced by humic matter, which causes a yellow-brown water colour. Colour is a physical indicator of the purity of surface waters. From an analytical point of view, water colour is a criterion for the establishment of the technological efficiency of a waterworks (Tesarik et al., 1987).
Aggregation of impurities by hydrolysing and organic coagulants is conditioned by their functional mechanisms (Polasek and Mutl, 2002a; Pivokonsky et al., 2011, 2020):
• Hydrolysing coagulants function by mechanisms for which the prerequisite for formation of a separable suspension is the destabilisation of particles of impurities, i.e., the energy barrier between colliding particles is either totally removed or suppressed by a simple electrolyte to such an extent that it can be forcibly overcome by the high kinetic energy of the colliding particles; the destabilised particles tend to collide, get attached one to another and form separable aggregates.
• Organic coagulants function by the mechanism of formation of inter-particle bridges between the particles of impurities and the polymer chains of molecules of organic coagulants. Due to this, the aggregate stability of the particles of impurities is not affected to an adequate extent. The particles of impurities are not destabilised. For this reason the water purification efficiency of organic coagulants is very poor; consequently organic coagulants are not an equivalent substitute for hydrolysing coagulants.
Based on personal experience, waterworks operating personnel and process design engineers do not seem to be aware of the different mechanisms by which organic and hydrolysing coagulants function and form aggregates, a functional difference which causes differing removal efficiencies for impurities in water.
Waterworks generally are not designed to accommodate both organic and hydrolysing coagulants. In comparison to a hydrolysing coagulant an organic coagulant necessitates an upgrade of the flocculation plant because considerably greater power input is required to complete aggregation (Polasek, 1980, 2011a,b). There is also a need to upgrade the filtration plant because of the more rapid build-up of head loss and the required higher velocities of air scour and water for backwashing the filter media. Based again on personal experience, the suppliers of organic coagulants do not appear to request waterworks management to provide such upgrades, possibly because they are not aware of these needs themselves or are not prepared to highlight the need for such upgrades.
In many South African waterworks using an organic coagulant, the process controllers are not allowed to carry out jar tests on a regular basis to determine optimum coagulant dosage. Jar tests can only be carried out by the organic coagulant supplier during his usual monthly visit; therefore process controllers adjust coagulant dosages based only on the visual appearance of the settled water with no verification of the impact of such dosage adjustment on the actual water quality.
In South Africa, the safe use of individual organic coagulants is usually certified by the national Department of Health. Such certification also prescribes the maximum permissible dosage. However, this does not certify the suitability of the product for the purification of water to a wholesome potable quality in terms of the National Standard, SANS 241:2015 (SABS, 2015). It merely certifies that the product is hygienically not objectionable within the limits of dosages stipulated in the certificate.
The concentration of NOM can be expressed by total and dissolved organic carbon (TOC, DOC) or by the chemical oxygen demand (COD). The TOC comprises DOC plus NOM particles which are naturally separable from water. The selection of water purification technology should be based on DOC.
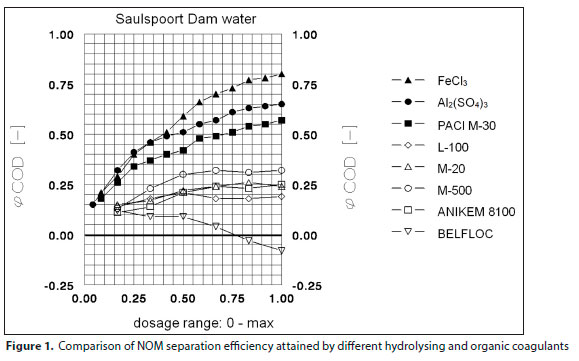
The process efficiency attainable by different mineral and organic coagulants, both synthetic and natural, are compared in Fig. 1 which is reproduced from Polasek and Mutl (2002a). In this figure, NOM was measured as CODMn; φCOD is NOM removal efficiency. The mineral coagulants are FeCl3, Al2(SO4)3 and M-30, which is polyaluminium chloride (PACl). The organic coagulants are polyamines - L-100 and M-20, polyDADMAC - M-500 and Anikem 8100, and Belfloc is a blend of Floccotan (a partially condensed product of commercial wattle tannin extract treated to produce active amine groups along the polymerised molecule) with polyDADMAC. Evidently, hydrolysing coagulants are capable of removing NOM with an efficiency greater than 70%. The efficiency of pure organic coagulants does not exceed 32% and some even increase the concentration of NOM in the purified water in comparison to that of the raw water. For this reason, organic coagulants should only be used for purification of waters which are only polluted with insignificant concentrations of NOM.
For the purposes of this article it is also necessary to emphasize that the particles of impurities consist of two fractions, namely, separable and non-separable particles (Hereit et al., 1977, Polasek 2014a). Total concentration of the monitored impurity measured in the raw water is designated as C0 and C0F, and in the filtrate as CF and CFF. The concentration C0 and CF means that the total concentration of such impurity is produced by all its particles. Abridger F means that the concentration of such impurity is produced by its non-separable particles only. The non-separable particles C0F and CFF are determined in the samples of tested water from which the separable particles have been removed by specific conditions of centrifugation (Polasek, 2014a, b). The total concentration of all particles of the monitored impurity remaining in the purified water determines the overall quality to which water is purified, whereas the concentration of its non-separable particles indicates the best attainable quality limit to which the water can be purified by the technology used.
MATERIALS AND METHODS
A Standard Phipp & Bird 6-station flocculator, maximum speed 100 r-min-1, complete with a fully adjustable, variable-speed controller, common to all 6 stations and a revolution counter, was used for jar tests.
Jar tests were carried out in 2-L standard Pyrex beakers with a 1.5-L volume of the raw water. A double paddle type stirrer of spinning diameter d = 62 mm and height h = 58 mm was used.
A Labofuge-1 laboratory centrifuge was used to remove the separable particles from tested samples in order to determine the content of the non-separable particles in the samples.
Turbidity was measured using a Hach 2100A turbidity meter. NOM was determined as TOC and DOC or CODMn. The TOC and DOC were analysed by Talbot & Talbot Water Laboratory using APHA (2005) Standard Methods. The CODMn were analysed by the authors with our colleague Dr Mutl using the modified Kubel (1866) method (Polasek and Mutl, 1995).
The jar tests are carried out with different organic and hydrolysing coagulants. These agents are dosed over a broad range of dosages. The dosage of each coagulant producing the lowest attainable residual turbidity, TOC and colour is the optimized dosage. In the case of hydrolysing coagulant it also includes its cation (Polasek and Mutl, 2005).
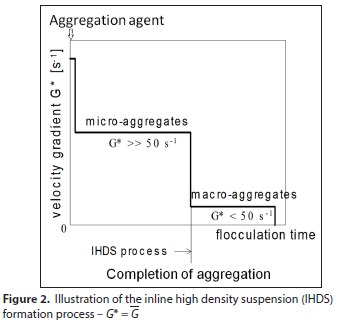
All jar tests comparing the effect of mineral and organic coagulants were carried out under the inline high density suspension (IHDS) formation process. The IHDS process shown in Fig. 2 took place at 100 r - min-1 until aggregation was completed and then continued at a low intensity agitation of 27 r - min-1 for a period of10 min to form larger, faster settleable flocs. The samples of water for determination of the purified water quality were taken after 60 min sedimentation.
RESULTS
Comparison of turbidity removal attained by mineral and organic coagulants
The effect of organic and hydrolysing coagulants on turbidity removal is compared in Table 1. Zetafloc 2350 (synthetic organic coagulant) and aluminium sulphate (mineral hydrolysing coagulant) were tested. Optimum dosages for turbidity removal by both coagulants were determined by jar tests. Turbidity produced by all particles of impurities and by non-separable particles was measured in the raw water and in the filtrate. The filtrates were produced by direct filtration of flocculated water in a pilot plant. Turbidity CF shows the residual filtrate turbidity and CFF the best residual turbidity attainable by the coagulant.
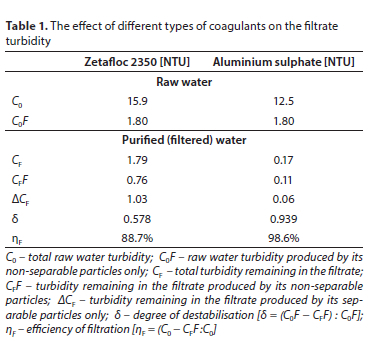
It is evident from the results in Table 1 that there are vast differences in the performance efficiencies of these different types of coagulants. The residual turbidity produced by the organic coagulant is CF-OC = 1.79 NTU, which far exceeds the filtrate turbidity produced by the hydrolysing coagulant, CF-HC = 0.17 NTU. The recommended limit for potable water is 0.3 NTU (APHA, 2005). Evidently the residual turbidity resulting from the use of the organic coagulant far exceeds this limit, whereas the residual turbidity resulting from the use of the hydrolysing coagulant is well below this limit. The separation efficiency for turbidity particles in the case of organic coagulant, <pTu, was 88.7%, whereas in the case of hydrolysing coagulant <pTu = 98.6%. The most important efficiency is that for the removal of the non-separable particles (Hereit et al., 1977; Polasek, 2014a) which determines the attainable quality of purified water. The residual turbidity resulting from the use of the organic coagulant is CFFOC = 0.76 NTU, whereas that resulting from the use of the hydrolysing coagulant is CFFHC = 0.11 NTU. This comparison indicates the performance efficiency attainable by the plant using these two types of coagulants. The process efficiency can also be described by the degree of destabilisation, δ. For the organic coagulant this is only φOC = 57.8%, in comparison to that for the hydrolysing coagulant which is φHC = 93.9%. In addition, the filtration efficiency attained by the organic coagulant is NF-OC = 88.7% and by the hydrolysing coagulant is Nf-hc = 98.6%. From the above it is evident that the organic coagulant purifies water to an inferior quality in comparison to the quality attained by the hydrolysing coagulant.
Capability of organic coagulants to remove NOM and turbidity from surface waters
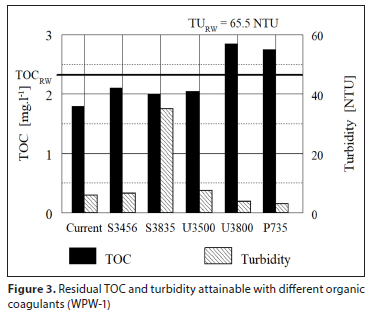
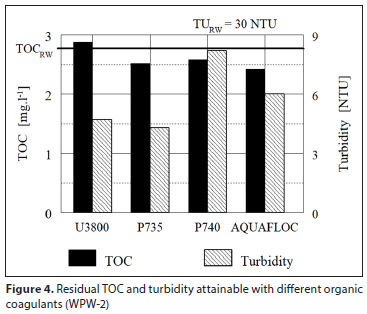
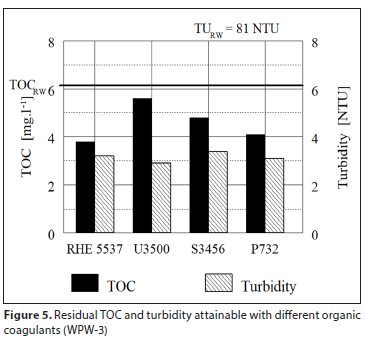
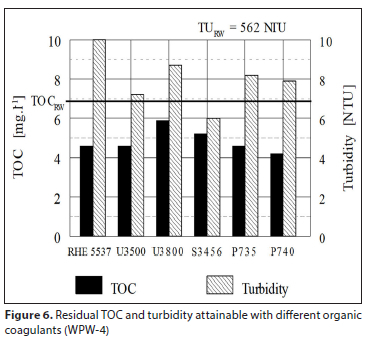
A supplier of organic coagulants in the Eastern Cape, South Africa, was asked to check the efficiency of different types of their products, by jar tests at 4 waterworks. Four to six different organic coagulants at their optimised dosages were tested. Purification efficiency attained by individual coagulants was evaluated by measuring TOC and turbidity in both the raw water and the purified water after 60 min sedimentation. The results obtained were measured for turbidity and TOC and processed graphically and are shown in Figs 3 to 6. The dosage of each coagulant producing the lowest residual turbidity and TOC is considered to be the optimum dosage. Intensity of agitation applied to jar tests and time of agitation was not reported.
Irrespective of the organic coagulant used, the results in Figs 3 to 6 clearly illustrate a low ability of organic coagulants for the removal of NOM; in some cases NOM concentration even increased in comparison to that of the raw water. Turbidity removal was also rather poor. Comparison of results between individual water purification works (WPW) in Figs 3 to 6 shows that the TOC removal efficiency φOC tends to increase with a higher raw water turbidity TuRW:
Generally, the chemical composition of individual organic coagulants is unknown to their users. This is because their suppliers consider the composition of organic coagulants to be trade secrets. Therefore, only the supplier's product trade name or trade number is known to the users.
NOM removal by various organic coagulants at different waterworks
TOC and DOC removal efficiencies attained by various organic coagulants under operational conditions at several Eastern Cape waterworks are shown in Table 2. The results obtained are expressed by percentage removal with respect to the composition of the raw water.
The design of all waterworks referred to in Table 2 is based on the concept of two-stage separation of formed suspension, i.e., chemical dosing, coagulation/flocculation, sedimentation, filtration and chlorine disinfection. The existing waterworks arrangement was originally designed for the use of a hydrolysing coagulant. The only change made was the replacement of the hydrolysing coagulant with a synthetic organic coagulant without any plant upgrade.
Waterworks A and B: The water is purified to within the best quality attainable by organic coagulants. There are two possible reasons for this: Either the most efficient organic coagulants are applied to waterworks which have an effectively designed purification system or the organic coagulant used is a blend of cationic polymer with hydrolysing coagulant.
Waterworks C and D: The organic coagulants resulted in a considerable increase in TOC and DOC in the purified water in comparison to that of the raw water. In the case of Waterworks D the reasons for such a great increase in both TOC and DOC is unknown.
Waterworks E to H: The reasons for the very low DOC removal efficiencies, of 13.3%, 18.7% and 14.4% are as follows: (a) the organic coagulants used, or (b) the waterworks system design, or (c) combination of both.
Waterworks I: It is evident from the high reaction pH of 9.1 that the coagulant, aluminium sulphate, was not applied under optimised reaction conditions. Therefore, a very low DOC removal efficiency of 31.4% and a high concentration of residual Al >1.5 mg-L-1 in the filtrate were achieved.
Waterworks J: Low reaction pH (4.6) produces high residual Al (1.1 mg-L-1) in the filtrate indicating aluminium sulphate was not applied under optimised reaction conditions. This condition very unfavourably affects the purified water quality and results in a poor DOC removal of 16.7% and high residual Al.
Waterworks K: The efficiency of DOC removal attained by FeCl3 under optimised reaction conditions was greater than 80%. When FeCl3 was replaced with organic coagulant (L-100) the DOC removal efficiency dropped to below 28%.
Mineral and organic coagulants in purification of highly eutrophic water
The purification of water at Waterworks L, described in Table 3, is very interesting. The raw water is highly eutrophic water of a Cape coastal type characterised by a very high colour (up to 800 HU), extremely high NOM (expected to reach CODMn > 70 mg O2-L-1) and low alkalinity, necessitating pre-alkalisation. The raw water is mono-polluted by heather. At the time of testing NOM in the raw water was high: CODMn = 52.5 mg O2-L-1 and colour Co = 505 HU.
Because of the softness of the water, aluminium sulphate was found to be the most efficient coagulant. When applied under optimised reaction conditions this water was purified to an excellent quality: CODMn was reduced to 1.64 mg O2-L-1 and colour to 4 HU. The optimised dosage, however, was fairly high, D = 165 mg-L-1. In comparison, ferric chloride was less efficient; CODMn was reduced to 2.75 mg O2-L-1 and colour to Co = 3 HU; its dosage D = 71 mg-L-1 is very cost effective. It is believed that increased pre-alkalisation of the raw water will allow the ferric chloride dosage to increase to a level of D ~ 100 mg-L-1 and this is expected to reduce CODMn < 2.5 mg O2-L-1.
It follows from Table 3 that the waterworks was not operated under optimised reaction conditions as the residual CODMn = 4.79 mg O2-L-1 is too high and well above the attainable value of 2.75 mg O2-L-1.
The application of organic coagulants (L-100, M-500) totally failed as their effect on the quality of purified water was unnoticeable.
Some side-effects of organic coagulants on filtration
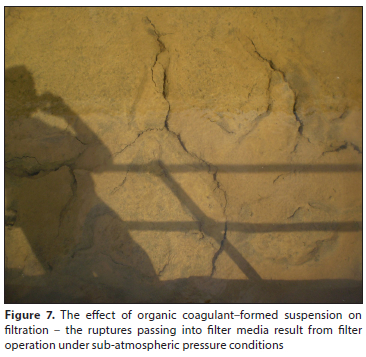
The adhesive (gluey) property of the suspension formed by organic coagulants very negatively affects the performance of rapid gravity filters. Their powerful attachment to the grains of filter media is evident from Figs 7 to 9. Figures 7 and 8 show the condition of filters approximately 6 months after installation of new filter sand.
The purpose of Fig. 7 is to show how the organic coagulant-formed suspension, due to its great adhesive properties, is retained at the top of the filter bed and causes a screening effect. This results in a rapid rise in filter head loss. Because the filter bed is designed with a low operational water head it also operates under an undesirable sub-atmospheric pressure condition (Hereit, 1969, 1973; Tucek et al., 1977; Polasek and Mutl 2002b; Hereit and Polasek 2014).
The stronger bond of aggregates formed by organic coagulant to the grains of filter media requires upgrading of the backwashing, either by employing a much greater intensity of air scour and backwash water than those initially designed for a hydrolysing coagulant-formed suspension or by converting backwashing to an air-plus-water combined method. This is necessary to maintain the required efficiency in the recovery of the sludge-holding capacity of the filter bed (filter clogging). Generally, upgrading of backwashing is not associated with the use of organic coagulants.
The effect of a too low intensity of air scour and backwash water, coupled with inappropriate filter geometry, is evident from Fig. 8. The filter in Fig. 8 is the Moore type and its geometry was designed by consulting engineers. The Moore type filter is characterised by a small capacity air blower which is used for two purposes. The first is air scour. The second, after completion of air scour, is to pressurise air in the filtrate tank below the filter to deliver water for filter backwashing. The consequences of this arrangement are obvious shortcomings restricting the attainable efficiency of backwashing.
Figure 8 also shows how the low efficiency of backwashing affects the next air scour and the backwashing efficiency in general. It was observed that within a very short time, of about 2 min, fairly clear backwash water was discharged from the filter bed, indicating to the operators that filter backwashing was completed. However the filter bed remains muddy and the efficiency of filtration together with the length of the filtration run continue to decrease.
It follows, from the above, that the prerequisite for the replacement of a hydrolysing coagulant by an organic coagulant is the requirement to upgrade the filtration plant in its complexity. Such upgrading should include the replacement of filter media with an adequately enlarged grain size together with an accordingly increased height of filter bed as well as upgrading of backwashing. The preferred upgrading involves the conversion to combined air-plus-water backwashing, which is the most effective and economic method. In addition, the operational height of the water level above the filter bed should be increased in order to prevent the filter bed from operating under sub-atmospheric pressure conditions (Polasek and Mutl, 2002b; Hereit and Polasek, 2014).
Regarding the need for a larger filter media grain size, Haarhoff (2014 p. 6) states: 'Filter sand within the range used in water treatment has the same porosity, or pore volume per volume of media bed. Whether a bed has finer sand (...) or coarser sand (...), the porosity or "sludge holding capacity"(...) is about the same.' Therefore, no greater grain size is required. Obviously such a statement is incorrect. It should be pointed out that porosity and sludge-holding capacity (filter clogging) are two different parameters. The sludge-holding capacity is dependent on the grain size (the size of voids between the grains) and not on the porosity, as this remains the same irrespective of grain size. For filtering of organic coagulant-formed aggregates the larger grain size is required to avoid accumulation of suspension on the top of the filter bed, as shown in Fig. 7.
Figure 9 illustrates how the organic coagulants facilitate formation of mudballs if the filter sand grain size is too small and filter backwashing is ineffective. This particular mudball originated from a pressure filter filtering settled water purified by an organic coagulant. This filter arrangement requires regular replacement of filter sand. At the time of sand replacement the settled water had to be pumped against 10 times the operational head loss compared to that of clean filter media. When the media was replaced, it was so compacted that the filter bed had to be mechanically broken into smaller chunks before filter sand could be bucketed from the filter. The severity of this problem is evident from one of these chunks, shown in Fig. 9.
DISCUSSION
It is evident from Table 1 that the organic coagulant does not remove turbidity to within the limit of 0.3 NTU recommended by the APHA (2005) standard for drinking water.
The results presented confirm that the NOM removal efficiency attainable by organic coagulants is incomparably lower than that of hydrolysing coagulants, i.e., <32% against >70% and therefore are unable to purify water for domestic supply to the quality required by the national standards of different countries, which are very similar to APHA (2005). This is the consequence of the different mechanisms by which hydrolysing and organic coagulants function.
The performance results obtained from different waterworks (Table 2) fully support the findings that the efficiency of organic coagulants for the removal of NOM is inferior in comparison to mineral hydrolysing coagulants. In the situation of water highly polluted with NOM (Table 3) and characterised by an extremely high colour (505 HU) and CODMn (52.5 mg O2-L-1), the purification effect of organic coagulants was unnoticeable, whereas the hydrolysing coagulant (aluminium sulphate) purified the water to an excellent quality: organic pollution was reduced to CODMn = 1.64 mg O2-L-1 and colour to Co = 4 HU, i.e., the efficiency of CODMn removal is NCODMn = 96.3% and colour is η€ο = 99.2%.
Evidently, organic coagulants are not equivalent substitutes for the traditional mineral coagulants. Therefore, the use of organic coagulants should be discontinued and the waterworks already using organic coagulants should be converted to the use of hydrolysing coagulants, applied under optimised reaction conditions.
Organic coagulant can be considered acceptable for use as a coagulant only if NOM in the raw water is of no technical significance, i.e., the recommended CODMn in the raw water does not exceed 2.5 mg O2-L-1, the organic coagulant used does not increase NOM in the purified water in comparison to that of the raw water, and all adverse side-effects influencing plant operation are addressed prior to its use, for instance flocculation and filtration plants.
Design of rapid gravity filters is generally based on filtering hydrolysing coagulant-formed suspensions. Since organic coagulant-formed suspension is characterized by a greater bond to the media grains, there is a need for upgrading of the filtration plant design accordingly. Usually, waterworks managers are not informed of this requirement. Therefore, deficiencies in the filter design and its backwashing are magnified by the use of organic coagulants. The upgrading of filters should be based on the filterability of the formed suspension (Hereit and Polasek, 2014).
The use of organic coagulants is quite popular even though they are very inefficient. Some of the reasons for their popularity can be identified as follows:
• The decision makers, waterworks designers, supervisors and process controllers are not provided with information that, unlike the mineral hydrolysing coagulants which remove the aggregate stability of the particles of impurities, the organic coagulants cannot do this because of different functional mechanisms. Therefore organic coagulants are not capable of effectively facilitating removal of hydrophilic colloids (NOM) and also some hydrophobic colloids from the purified water to a level that meets the requirements of the national standards of various countries for water for domestic supply.
• The use of organic coagulants does not change the pH of the water, which makes them user-friendly. The fact that these coagulants do not purify water to a wholesome quality is most probably unknown to the waterworks process controllers, decision makers and general public.
• Broad acceptance of organic coagulants in certain countries worldwide, together with numerous articles supporting their use, provide the necessary comfort to the users of organic coagulants. In addition, the waterworks process controllers and decision makers are not provided with a simple explanation about the inability of organic coagulants to purify water to a healthy risk-free wholesome quality in terms of their national standards. Therefore, they cannot see the reasons why organic coagulants should not be used and the need for their replacement with hydrolysing coagulants.
Determination of NOM by TOC and DOC is a fairly complex and lengthy analysis. Based on experience it is not in the capability of most waterworks to carry out such analysis. Therefore, these determinants cannot be used as the routine operational control parameters for optimising and establishing the performance efficiency of waterworks. Instead, the chemical oxygen demand (CODMn), measured by the Kubel method as oxidizability, is used in many countries as the routine operational indicator of organic pollution. If oxidation of analysed water takes place under boiling conditions it is marked CODMn. It is a very simple and short method that can be used at any waterworks. This analysis is completed within 30 min and can be carried out by properly trained process controllers.
The results in Figs 1 to 9 are absolutely conclusive in that organic coagulants are not equivalent replacements for hydrolysing coagulants under any circumstances.


CONCLUSIONS
Based on the above results, the following conclusions can be drawn:
• Organic coagulants are ineffective for the removal of NOM as well as turbidity and have adverse effects on the operation of rapid gravity filters.
• Purification of water to its best health-risk-free quality, meeting the requirements of the National Standard, is not attainable with organic coagulants.
• Failure to remove NOM can lead to both the formation of trihalomethanes after chlorination and to secondary pollution of water in the distribution network.
• The adverse side effects on rapid gravity filters require their upgrading. This includes:
• Adequate enlargement of media grain size and the filter bed height to be increased accordingly
• Upgrading of filter backwashing, preferably by combined air and water, in order to efficiently and economically achieve the required recovery of the sludge-holding capacity of the filter bed
• Appropriate increase of operational water height above filter bed
• Modification of filter geometry to ensure fast and efficient removal of suspensions washed out from the filter bed
• TOC and DOC analysis are very complex and time consuming. Therefore, they are not suitable routine monitoring parameters for NOM removal at waterworks. Instead it is recommended to use CODMn because it is a very simple and fast method which can be carried out at any waterworks.
• The maximum acceptable concentration of TOC/DOC in the purified water is specified in the national standards for potable water of various countries. If CODMn is not specified in the national standard then its maximum concentration of 2.5 mg O2-L-1 is recommended.
• Organic coagulants should be used only when NOM concentration in the raw water is not of technical significance.
• Process controllers should be obliged to carry out jar tests to determine optimum dosage of coagulant at least once a day.
NOMENCLATURE
Co colour
COD chemical oxygen demand
CODMn chemical oxygen demand determined by Kubel method
DOC dissolved organic carbon
HU Hazen units
IHDS inline high density suspension formation process
NOM natural organic matter
OC organic coagulant
TOC total organic carbon
φCOD COD removal efficiency in %
φTu turbidity removal efficiency %
REFERENCES
AMRAN AH, ZAIDI NS, MUDA K and LOAN LW (2018) Effectiveness of natural coagulant in coagulation process: a review. Int. J. Eng. Technol. 7 (3.9) 34-37. https://doi.org/10.14419/ijet.v7i3.9.15269 [ Links ]
APHA (2005) Standard Methods for the Examination of Water and Wastewater (21st edn). American Public Health Association/ American Water Works Association/Water Environment Federation, Washington DC. [ Links ]
HAARHOFF J (2014) Alleged filtration defects at Inyaka Water Treatment Plant. Expert report prepared for Bigen Africa and presented to Department of Water Affairs, South Africa. [ Links ]
HEREIT F (1969) Obracene protekane filtry. [Filters with reverse flow]. PhD thesis, CVUT Praha. [ Links ]
HEREIT F (1973) Filtrace vody ve vodarenstvi [Water filtration in water management]. Methodical Information No. 6. MVLH, CSR, Praha. [ Links ]
HEREIT F, MUTL S and VAGNER V (1977) Hodnoceni provozu upraven vody [Evaluation of waterworks performance]. J. Vodni Hospodarstvi-B 27 (4) 80-86. [ Links ]
HEREIT F and POLASEK P (2014) Methodology for evaluating and monitoring of waterworks performance efficiency - Part 2: Test of filterability. Adv. Chem. Eng. Sci. 4 470-482. https://doi.org/10.4236/aces.2014.44049 [ Links ]
HOCMAN G (1986) Chemistry and Carcinogenicity [in Slovak]. Alfa, Bratislava. [ Links ]
KUBEL W (1866) Anleitung zur Untersuchung von Wasser [Instructions for studying water]. Vieweg um Sohn, Braunachweig. [ Links ]
PITTER P (1981) Hydrochemie [Hydrochemistry]. SNTL, Praha. [ Links ]
PIVOKONSKY M, BUBAKOVA P, PIVOKONSKA L and KNESL B (2011) Tvorba Suspense pri Uprave Vody; Teorie a Praxe [Formation of suspension at water purification; theory and practice]. Sovak CR, Praha. [ Links ]
PIVOKONSKY M, VASATOVA P, NACERADSKA J and PIVOKONSKA L (2020) Koagulace pri Uprave Vody, Teorie a Praxe [Coagulation at water purification, theory and practice]. Academia, Praha. [ Links ]
POLASEK P (1980) Methods and testing procedures for monitoring and evaluating waterworks performance. Proc. Int. Conf. on Filtration and Separation, SAFIL, Johannesburg. [ Links ]
POLASEK P (2011a) Influence of velocity gradient on optimisation of the aggregation process and physical properties of formed aggregates: Part 1. Inline high density suspension aggregation process. J.Hydrol. Hydromech. 59 (2) 107-117. https://doi.org/10.2478/v10098-011-0009-5 [ Links ]
POLASEK P (2011b) Influence of velocity gradient on optimisation of the aggregation process and physical properties of formed aggregates: Part 2: Quantification of the influence of agitation intensity and time of the properties of formed aggregates. J. Hydrol. Hydromech. 59 (3) 196-205. https://doi.org/10.2478/v10098-011-0016-6 [ Links ]
POLASEK P (2014a) Methodology for evaluating and monitoring of waterworks performance efficiency - Part 1: Methods and testing procedures. Adv. Chem. Eng. Sci. 2014 (4) 208-220. https://doi.org/10.4236/aces.2014.42024 [ Links ]
POLASEK P (2014b) Significance and determination of portion of non-separable particles of impurities in water purification. Water SA. 40 (1) 89-94. https://doi.org/10.4314/wsa.v40i1.11 [ Links ]
POLASEK P and MUTL S (1995) Guidelines to coagulation and flocculation for surface waters, Volume 1: Design principles for coagulation and flocculation systems. PPA, Johannesburg. [ Links ]
POLASEK P and MUTL S (2002a) Cationic polymers in water treatment, Part 1: Treatability of water with cationic polymers. Water SA 28 (1) 69-82. https://doi.org/10.4314/wsa.v28i1.4870 [ Links ]
POLASEK P and MUTL S (2002b) Cationic polymers in water treatment, Part 2: Filterability of CPE-formed suspension. Water SA 28 (1) 83-88. https://doi.org/10.4314/wsa.v28i1.4871 [ Links ]
POLASEK P and MUTL S (2005) Optimisation of reaction conditions of particle aggregation in water purification - back to basics. Water SA 31 (1) 61-72. https://doi.org/10.4314/wsa.v31i1.5122 [ Links ]
SABS (South African Bureau of Standards) (2015) SANS 241:2015. South Africa National Standard 2015. Drinking Water. Part 1: Microbiological, Physical, Aesthetic and Chemical Determinands. SABS, Pretoria. [ Links ]
TESARIK I, LATAL M, OSLEJSEK J, PELIKAN V, PIVODA B, ROZKYDALEK J, SEREK M and VOSTRCIL J (1987) Vodarenstvi [Water management]. SNTL/ALFA, Praha. [ Links ]
TUCEK J, CHUDOBA F and KONICEK Z (1977) Zakladni Procesy a Vypocty v Technologii Vod (Basic processes and calculations in water technology). SNTL, Praha. [ Links ]
 Correspondence:
Correspondence:
P Polasek
Email: pavelpolasek40@gmail.com
Received: 4 May 2021
Accepted: 30 June 2023














