Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
Water SA
versão On-line ISSN 1816-7950
versão impressa ISSN 0378-4738
Water SA vol.49 no.3 Pretoria Jul. 2023
http://dx.doi.org/10.17159/wsa/2023.v49.i3.4008
RESEARCH PAPER
The effect of the linear alkylbenzene sulfonate, sodium-dodecyl-sulfonate (SDS), on the bioaccumulation of Al, Sr and Mn by Brassica oleracea and Solanum tuberosum
Glynn K PindihamaI; Mugera W GitariI, II
IEnvironmental Remediation and Nano Sciences Research Group, Department of Geography & Environmental Sciences, Faculty of Science, Engineering and Agriculture, University of Venda, South Africa
IIDepartment of Chemical Sciences and Technology, School of Chemistry and Material Sciences, Technical University of Kenya, Nairobi, Kenya
ABSTRACT
The hyper-eutrophic conditions in impoundments used for irrigation around South Africa's major cities promote the co-existence of linear alkylbenzene sulfonate (LAS) and other pollutants such as metals. The combined effects of LAS and metals, when such water is used to irrigate crops, has not been properly investigated in light of human health risks and prevailing local conditions. To understand the potential risks, pot-culture experiments were conducted to assess the effect of the LAS, sodium-dodecyl-sulfonate (SDS), on the accumulation of aluminum (Al), manganese (Mn) and strontium (Sr) in Brassica oleracea (cabbage) and Solanum tuberosum (potato) plants. The plants were watered with dam water containing 3.48 mg-L-' of the LAS (sodium dodecyl sulfonate) and Mn (0.257 mg-L-'), Al (0.6 mg-L-') and Sr (0.16 mg-L-') as determined by field surveys, for 20 days. The presence of SDS in the irrigation water at environmentally relevant concentrations did not enhance uptake of Sr, Mn, Al in the two plants, as demonstrated by statistically insignificant differences in the means of the treatments (with and without SDS). In addition, the presence of the metals, high pH, EC and presence of cyanotoxins in the water did not affect total chlorophyll and growth of the plants. These findings imply that the prevailing levels of anionic surfactants such as SDS, metals and other contaminants in the hyper-eutrophic reservoirs pose little risk to crop yields, quality of crops and human health, due to the possible accumulation of these contaminants in irrigated plants. Despite the study reporting no immediate inherent risk to the plants and human health, continuous monitoring of the contaminants in water, soil and irrigated plants is recommended since the conditions, concentrations and other factors can quickly change if the management of the catchment does not improve in the near future.
Keywords: Brassica oleracea, combined effects, sodium-dodecyl-sulfonate, metals, Solanum tuberosum
INTRODUCTION
South Africa is known for having scarce and extremely limited water resources and depends mainly on surface water for its urban, industrial and irrigation requirements. Metal contaminants in soil have the ability to migrate and accumulate (Sulaiman and Hamzah, 2018). The accumulation of metal contaminants in soil is of concern, since these can affect the well-being of plants, animals, and humans. In plants, increased levels of metals can induce oxidative stress and also hinder the plants' ability to produce chlorophyll (Sulaiman and Hamzah, 2018).
Linear alkylbenzene sulfonates (LAS) belong to a group of anionic surfactants commonly used in domestic and industrial processes (Wang et al., 2015). Anionic surfactants, in particular, are a common ingredient in detergents due to their simple synthesis and low cost (Pierattini et al., 2018). LAS find their way into the aquatic environment through the discharge of untreated and treated wastewater. LAS elimination in the aquatic environment is via adsorption and biodegradation, but their degradation is very slow in anaerobic and anoxic environments and this leads to their accumulation under such conditions in water (Wang et al., 2012). This makes hypereutrophic lakes and reservoirs ideal environments for the co-existence of toxic cyanobacteria, LAS and other pollutants, since the excessive growth of cyanobacteria in eutrophic lakes consumes oxygen and their eventual death and degradation makes water bodies anoxic and anaerobic. Previous studies have looked into the synergic impacts of LAS with other contaminants such as metal pollutants, pyrene and oil (Wang et al., 2012).
In South Africa (SA), dams like the Roodeplaat and Hartbeespoort, which are found in the Gauteng and North West Provinces, respectively, are renowned as hyper-eutrophic and having poor water quality (Pindihama and Gitari, 2020). The co-existence of LAS and other pollutants such as microcystins (MCs) and metals thus require examination, since water derived from these dams is mainly used for irrigation. The aim of this study was to assess the effect of the LAS, sodium-dodecyl-sulfonate (SDS), on the accumulation of the metals aluminum (Al), manganese (Mn) and strontium (Sr) in Brassica oleracea (cabbage) and Solanum tuberosum (potato) plants when exposed to environmentally relevant concentrations of the pollutants.
MATERIALS AND METHODS
Materials and reagents
A field survey was conducted in June 2019 and September 2019 to identify and collect field water suitable for the experiments. The water was collected from canals and farm dams from the two sites:
Roodeplaat Dam and Hartbeespoort Dam. Total dissolved solids (TDS), electrical conductivity (EC), pH and turbidity of the water were monitored in-situ, and anionic surfactants, chlorophyll a, microcystins (MCs) and cations were measured ex-situ. The water was kept frozen at -20°C until required. The LAS used in this study was sodium dodecylbenzene sulfonate (SDS) (CAS No. 25155-30-0; molar mass 348.48 g-mol-1; and chemical formula C18H29NaO3S, acquired from BYMAZ Pty Ltd, Johannesburg, South Africa).
Pot-culture experimental design
The Brassica oleracea seeds were purchased from NTK Agricultural Products & Services (SA) and the Solanum tuberosum seeds were purchased from Livingseeds Heirloom Seeds (Pty) Ltd Midvaal, Gauteng. All the S. tuberosum seeds were first washed with distilled water before being planted in 200 mm plant pots filled with uncontaminated soil. The B. oleracea seedlings were produced and pre-grown in plastic trays with uncontaminated soil. The soil used in this study was collected from the agricultural farm at the University of Venda. The farm lies within the Lowveld climatic zone and has well-drained deep red soils mostly dominated by clay; the soil falls in the Hutton classification which is the same as the Rhodic Ferralsol (Mabasa, 2019). The background levels of metal elements in the soil used in the pots are presented in Table 1. The metal elements in the soils were extracted and determined as described in the section on 'digestion of plant and soil samples'. With regards to the three main nutrients, P, K, total N and organic matter, the soils were analysed at the South African Agricultural Research Council. The organic carbon was analysed using the Walkley-Black method, P was extracted using the ISFEI method as described by Manson and Roberts (2001) and the extract was determined via the molybdenum method using an auto-analyser. Exchangeable and soluble K was extracted using the procedure described by Manson and Roberts (2001) and the K in the leachate was determined by atomic absorption spectrophotometry. Total N in the soil was determined using the wet oxidation procedure, commonly known as the Kjeldahl distillation, as described by Manson and Roberts (2001). P, K, total N and organic matter in the soils were 25.86 mg-kg-1; 184 mg-kg-1; 0.079% and 2.07%, respectively, which are typical of agricultural soils (FAO, 2015). The soil was collected from a depth of 0-50 cm, and approximately 15 kg of the soil was placed into 200 mm plastic pots for the experiments and treated with 6 g of Protek General Fertilizer with N:P:K (%) 2:3:2 (14) before introducing the plants.
To investigate the effect of the LAS sodium-dodecyl-sulfonate (SDS) on metal (Mn, Al and Sr) accumulation in B. oleracea and S. tuberosum, plants were watered daily with Roodeplaat Dam water containing 3.4 mg-L-1 of SDS (as determined by the field study), a known concentration of microcystins (± 15 μg L-1), and fixed levels of Mn (0.257 mg-L-1), Al (0.6 mg-L-1) and Sr (0.16 mg-L-1) as established from the field study (Table 3) for 20 days. During the field surveys irrigation water from the Roodeplaat Dam was monitored twice over a 4-month period to determine levels of a range of metallic elements (Table 3). Three elements, Al, Mn and Sr, were consistently detected, and the highest concentrations reported for the three elements were applied in order to investigate the worst-case scenario. The experimental design showing the 4 treatments the plants were exposed to is presented in Table 2. Treatment 1 consisted of milli-Q water (without any contaminants). Treatment 2 was raw dam water with the metals under investigation but without SDS. Treatment 3 consisted of milli-Q water and SDS (refreshed daily) but without the metals. Treatment 4 was raw dam water with the metals and SDS (refreshed daily to maintain constant SDS levels).
In order to maintain approximately constant concentrations of the SDS, the media were tested daily using a Hanna HI96769 anionic surfactants portable photometer, and refreshed accordingly. The accumulation of metals was determined in B. oleracea after 5 days and again after 20 days. Metal accumulation in S. tuberosum was determined only after 20 days. The total chlorophyll was determined in leaves of both plants at Day 20.
Determination of total chlorophyll
Reactive oxygen species (ROS) generation in a stress environment in plants causes changes in chlorophyll, anthocyanin and membrane integrity, among other effects; therefore, ROS generation can be measured indirectly by measuring the changes in these compounds (Venkidasamy et al., 2019). Chlorophyll content was measured according to Baskar et al. (2015). In brief, 50 mg of the plant leaves was sliced into small pieces and soaked in 95% (v/v) ethanol and then incubated for 3 days in the dark. The absorbance of the supernatant was read at 664.2 and 648.6 nm by UVvis spectrophotometer (SPECTROstar Nano, BMG LABTECH, Germany). Chlorophyll a and b and total chlorophyll content were calculated according to Baskar et al. (2015) using the following formulae:

Total chlorophyll content was expressed as milligram per gram of fresh matter (FM).
Digestion of plant and soil samples
Both soil and plant samples were digested according to Rashid et al. (2016). In brief, soil samples were dried for 24 h at 60°C in an oven then ground into a fine powder using a mortar and pestle. The ground soil samples (5 g) were then transferred into a 250 mL conical flask, and 10 mL of aqua regia (HCl:HNO3 (3:1)) was added. A hot plate was used to digest the samples at 95°C for 1 h; then left to cool to room temperature. The samples were diluted with deionized water and the supernatant filtered through Macherey-Nagel No.1 filter paper (0.45 μm), Macherey-Nagel, Germany, before analysis with ICP-MS.
The edible parts of the plants (leaves for B. oleracea and tubers for S. tuberosum) were first freeze dried for 48 h at -54°C under a constant vacuum of 44 μmHg (Telstar Lyoquest Freeze Dryer, Terrassa, Spain). The freeze-dried material was ground to powder using mortar and pestle, and 1 g of the ground material was mixed with 10 mL of HNO3 and allowed to stand overnight before being digested on a hot plate until the solution was semi-dry. The mixture was cooled and filtered through Macherey-Nagel No. 1 filter paper (0.45 μm), Macherey-Nagel, Germany, and then diluted with deionized water to the mark in a 50 mL volumetric flask and then sent to the Stellenbosch University Central Analytical Facility for inductively coupled plasma mass spectrometry (ICP-MS) analysis.
Determination of cyanobacterial biomass and microcystins
To determine chlorophyll a levels, hot ethanol extraction followed by spectrophotometric analysis of absorbency wavelength on a Spectro-star Nano (BMG LABTECH, 601-1106, Germany) according to Lawton et al. (1999) was used. Briefly, absorbency was monitored at 665 and 750 nm wavelengths and second readings were taken upon acidifying the same samples with 10 μL of hydrochloric acid (1 mol-L-1) at the same wavelengths to correct for turbidity. The corrected absorbance and turbidity at 750 nm was subtracted from 665 nm absorbance before and after addition of hydrochloric acid. The total chlorophyll a was determined according to the following formula provided by Lawton et al. (1999):
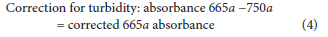
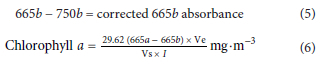
where: Ve = volume of ethanol extract (mL); Vs = volume of water sample (L); I = path length of cuvette (cm)
Levels of microcystins (MCs) in the Roodeplaat Dam water used were determined using the commercially available enzyme-linked immunosorbent assay (ELISA) microcystin plate kits (Envirologix Inc. (Kit Lot: 071499 Cat No: EP 022)). This assay uses antibodies against microcystins and a microplate reader Spectro-star Nano (BMG LABTECH, 601-1106, Germany) was used to quantify the MCs after the assay. Prior to analysis, 5 mL of each sample was filtered using the 0.20 μm glass fibre syringe filters and 50 μL of the filtered sample was used for the assay.
Data analyses
To compare the levels of accumulated cations and the total chlorophyll of the various plant treatments, analysis of variance (ANOVA) and/or Kruskal-Wallis tests were used at p < 0.05 using GraphPad InStat 3 (GraphPad Software, California, United States). Levels of cations are presented by their means ± the standard deviation (SD). Kolmogorov-Smirnov and Bartlett tests were used to test for normality and variance homogeneity at p < 0.05. Data which passed this test were compared using ANOVA and data which did not pass that test was compared using the Kruskal-Wallis at p < 0.05. The Tukey-Kramer multiple comparisons test and the Dunn's multiple comparisons test were used as post-hoc assays for data which passed the normality tests and data which did not pass the normality test, respectively.
RESULTS
Physicochemical parameters of the dam water
Dam water used to water the plants was alkaline, with mean pH of 9.02 (± 0.29), high EC and TDS levels (380 ± 16.52 μS-cm-1 and 228 ± 7.51 mg-L-1, respectively). The water also had a high cyanobacterial biomass (chlorophyll a 440.24 ± 328.147 μg L-1) and high MC levels (13.03 ± 3.599 μg L-1). The pH of the dam water used for irrigation was above the 6.5-8.4 threshold for water intended for irrigation in SA (DWAF, 1996). Even though the EC of the dam water was quite high, it was within the SA (DWAF, 1996) and Food and Agriculture Organization (FAO) (1985) (Ayers and Westcot, 1985) limits for irrigation water of < 400 μS-cm-1 and 700 μS-cm-1, respectively. The levels of anionic surfactants in the water ranged from 0.13 to 3.4 mg-L-1.
Table 3 shows the levels of cations in the raw dam water. All the cations in the dam water were within the SA (DWAF, 1996) and FAO (1985) guidelines for irrigation water. Metals such as Sr, Mn and Al were detected at significant levels in the dam water; hence their selection for the pot culture experiments.
Accumulation of Al, Sr, Mn and other cations by B. Oleracea in the presence of SDS
Findings from the pot-culture experiments in Table 4 show that upon 20 days of exposure to the various treatments, the B. oleracea leaves accumulated Mn to a maximum of 69.79 (± 22.97) μg-kg-1 (Treatment 2) and a minimum of 57.69 (± 12.52) μg-kg-1 (Treatment 1). Sr was accumulated up to 127.98 (± 26.60) μg-kg-1 (Treatment 3), with a lowest accumulation of 126.22 (±28.26) μg-kg-1 (Treatment 2). The highest levels of Al were accumulated in Treatment 1 at 0.18 (± 0.04) mg-kg-1, and the lowest levels were accumulated in Treatments 3 and 4, at 0.15 (± 0.04) mg-kg-1. The findings indicate no significant differences in the accumulated levels of Mn, Sr and Al in B. oleracea leaves after 5 days of exposure and after 20 days of exposure (ANOVA,p > 0.05) (Fig. 1).
A comparison of the accumulated metals among the 4 treatments presented in Fig. 2 and Table 4 shows that no significant differences were observed among the treatments for Sr and Mn (p > 0.05). Significantly higher levels of Al were accumulated in plants exposed to Treatment 2 (T2, dam water containing the three metals) compared to the other treatments (p < 0.05). This implies that accumulation of Al in B. oleracea was not affected by the presence of the SDS in Treatment 3 and combined exposure of metals and SDS in Treatment 4.
With regards to the 19 other major and trace cations also assessed in Table 4, only Cr, Co, Mg and P showed a significant difference in the levels accumulated among the 4 treatments (p < 0.05). For Co, Cr and P, much higher levels were accumulated in plants exposed to Treatment 1 compared to the other treatments, and for Na, plants exposed to Treatment 2 accumulated higher levels of the cation.
Accumulation of Al, Sr, Mn and other cations by S. tuberosum in the presence of SDS
Table 5 and Figure 3 shows the accumulation of the metals in the S. tuberosum tubers. Higher levels of Mn were accumulated for Treatment 3 (17.34 ± 4.93 μg kg;-1) and lowest levels for Treatment 1 (11.07 ± 2.85 μg kg-1). For Sr, higher accumulation was also for Treatment 3 (4.73 ± 0.91 μg·kg-1) and lowest for Treatment 1 (2.93 ± 0.38 μg·kg-1). Accumulation of Al was highest for Treatment 3 (0.17 ± 0.06 μg kg-1) and lowest for Treatment 4 (0.10 ± 0.05 μg kg-1).
Accumulation of Mn and Sr was much higher in the edible parts of B. oleracea (leaves) compared to the edible parts (tubers) of S. tuberosum plants (highest Mn accumulation of 69.79 (± 22.97) in B. oleracea and 17.34 (± 4.93) in S. tuberosum and highest accumulation for Sr of 127.98 (± 26.60) in B. oleracea and 4.73 (± 0.91) in S. tuberosum). The accumulation of Al was comparable in the edible parts of the two plant species (maximum accumulation of 0.18 (± 0.04) in B. oleracea leaves and 0.17 (± 0.06) in S. tuberosum tubers).
There were no significant differences in the accumulation of Al and Mn in the S. tuberosum tubers (p > 0.05), but significantly different accumulations were found for Sr (p < 0.05), with Treatment 1 having a significantly lower uptake compared to the other three treatments. Since plants exposed to Treatment 1 were not exposed to any SDS nor metals, this probably explains the lower levels of Sr accumulated by the tubers in Treatment 1. With regards to the other major and minor cations also assessed in the tubers (Table 5), no significant differences in the accumulations were found for all the other cations assessed (p > 0.05).
Effects of Al, Sr, Mn and other cations on B. Oleracea tolerance and S. tuberosum
Since increased levels of metals in plants are known to induce oxidative stress and also hinder the plants' ability to produce chlorophyll (Shakya et al., 2008; Sulaiman and Hamzah, 2018), we monitored total chlorophyll levels in the two plant species to assess the potential effects of the Al, Sr, Mn and other cations on the plants. In addition, the dam water used in Treatments 2 and 4 also had significant levels of microcystins (MCs) (15 ± 3.88 μg L-1), high pH (9.02 ± 0.29), high EC levels (380 ± 16.52 μs·cm-1) and high TDS levels (228 ± 7.51 mg-L-1). All these contaminants, i.e. MCs (Saqrane et al., 2008; Machado et al., 2017), high pH and EC (Huang et al., 2017) and anionic surfactants (Pandey and Gopal, 2010; Wang et al., 2012) are also known to induce oxidative stress, reduce chlorophyll production and affect plant growth.
Figure 4a shows comparable total chlorophyll content in the leaves of B. oleracea plants exposed to Treatment 1 compared to other treatments (no statistically significant differences in the mean total chlorophyll content among the plants exposed to the 4 treatments after 20 days of exposure (ANOVA, p > 0.05)). With regards to the total chlorophyll content of the S. tuberosum leaves, comparable total chlorophyll levels were observed in plants exposed to Treatments 1 to 4 (no statistically significant differences in the mean total chlorophyll levels among the 4 treatments; ANOVA, p > 0.05). The findings imply that exposure to environmentally relevant levels of the 3 metals and SDS as applied in this study, and the presence of other major and trace cations and MCs in the raw dam water did not induce oxidative stress nor inhibit chlorophyll production in the plants. In addition, no significant visual impacts were observed on the plants exposed to the 4 treatments.
DISCUSSION
Pollution of aquatic ecosystems and soils by anionic surfactants is common due to their widespread use in soaps and detergents globally. The presence of anionic surfactants in the dam water collected from Roodeplaat and Hartbeespoort Dam sites was confirmed. Levels of anionic surfactants found in the water (0.13 to 3.4 mg-L-1) were within the range (0.001 and 20 mg-L-1) generally found in surface waters (Wang et al., 2015). Both dams are considered hyper-eutrophic and warm monomictic impoundments (Van Ginkel, 2004) and the long history of mining, industrial activities and a rapidly growing urban population in the catchments where these two dams are found promotes the co-existence of pollutants such as anionic surfactants LAS, cyanotoxins and metals in these two dams (Pindihama and Gitari, 2020).
Some of the contaminants observed in the dam water used to irrigate the plants in Treatments 2 and 4 have been reported to have adverse effects on plants, e.g., MCs are known to induce oxidative stress (Saqrane et al., 2008; Machado et al., 2017), high pH and EC are known to induce oxidative stress and affect chlorophyll production (Huang et al., 2017), and anionic surfactants like LAS are also known to induce oxidative stress, reduce chlorophyll production and affect plant growth (Pandey and Gopal, 2010; Wang et al., 2012).
In this study, exposure to LAS in the form of SDS at relevant environmental concentrations did not affect the total chlorophyll of the plants. Previous studies have reported improved plant growth due to exposure to LAS in the range 0.3-10 mg-L-1, and significant stunted growth when the common aquatic duckweed (Lemna minor) was exposed to 20-30 mg-L-1 SDS (Wang et al., 2012).
Anionic surfactants like SDS are amphipathic compounds and can easily interact with the polar and non-polar components of cell membranes, resulting in membrane damage, and induce oxidative stress (Forni et al., 2012; Pierattini et al., 2018). Toxic effects such as reduced phenols and chlorophyll content and increased activity of stress-related enzymes upon exposure to SDS have been reported in aquatic plants like L. minor (Wang et al., 2012; Forni et al., 2012) and Azollapinnata (Pandey and Gopal, 2010). In the current study, the plants were exposed to much lower levels (± 3 mg-L-1) of SDS compared to those reported in previous studies (> 10 mg-L-1) (e.g. Pandey and Gopal, 2010; Wang et al., 2012; Forni et al., 2012); this may be the reason that no significant impacts on total chlorophyll were reported between the treatments and the control plants.
The presence of LAS in the irrigation water, in the form of SDS at environmentally relevant concentrations, did not enhance uptake of Sr, Mn, Al in the two plants tested here, as demonstrated by statistically insignificant differences in the means of the four treatments. In the case of S. tuberosum, where a statistically significant difference was observed for Sr, low uptakes were reported in the control plants, but plants exposed to metal-containing dam water without any SDS, plants exposed to milli-Q water with SDS (without any metals) and plants exposed to dam water with SDS and containing the three metals (Sr, Mn and Al), showed no differences. This implied that the accumulation of Sr in the S. tuberosum tubers was not affected by the presence of SDS and was independent of the presence of the metal in the water used for irrigation.
The presence of SDS also did not affect the uptake and accumulation of Sr, Mn, Al and 19 other major and trace cations in B. oleracea. Statistically significant higher accumulation of Al in Treatment 2 (dam water spiked with Sr, Mn and Al) compared to other treatments, particularly Treatments 3 and 4 which had SDS, implies that SDS at the levels tested (3.4 mg-L-1) did not enhance the uptake of the metal by the plants. With regards to other cations which were not spiked in any of the treatments, but were initially present in the dam water and soils used, significantly higher accumulations of Cr, Co, Mg and P in Treatments 1 and 2, which did not have any SDS, also supported the finding that uptake and accumulation was independent of the presence of SDS.
Previous studies reported contrasting results on the uptake of metals in the presence of anionic surfactants like the LAS, SDS. Hasan et al. (2019) reported increased Cd accumulation in shoots and roots of Althaea rosea upon exposure to 348.48 mg-L-1 of SDS. In another study, Pierattini et al. (2018) did not report significant total accumulation of Zn by poplar plants Poplus alba, but observed increased accumulation in leaves when 1 mM Zn was applied in combination with 0.5 mM of SDS compared to when 1 mM Zn was applied alone. Pierattini et al. (2018) also reported increased translocation of Zn from roots to leaves when the poplar plants were exposed to SDS. Contrary to Pierattini et al. (2018), Almeida et al. (2009) found that the LAS, SDS enhanced Cu accumulation in the salt marsh plant Halimione portulacoides, but did not find any Cu translocation to the other parts of the plant.
Consistent with our findings, Zhang et al. (2008) reported a reduction in Cd uptake by soybean plants in the presence of LAS. Zhang et al. (2008) found a reduction in Cd bound to carbonates and exchangeable Cd in the soils when the soils are exposed to LAS, hence the low uptakes reported. Almeida et al. (2009) did not find any influence of LAS on Cu levels in sediments. In addition to data suggesting little influence of anionic surfactants on the solubility of metals, Hasan et al. (2019) and Mao et al. (2015) reported degradation of LAS by strains of Pseudomonas, which use the contaminant as a source of carbon. The presence of such bacteria (Pseudomonas) to degrade the LAS in the soils in the study area was highly likely, given the climate (average annual temperatures ranging between 14°C and 29°C) in the study area. In addition the levels of LAS applied here were much lower compared to those applied in studies where significant metal uptakes were reported (e.g. Pierattini et al., 2018; Hasan et al., 2019).
At low concentrations, surfactants build up at liquid to liquid or at solid to liquid interfaces as monomers (Mao et al., 2015). Increasing their concentrations eventually replaces the interfacial solvent, such as water, leading to decreased polarity of the aqueous phase and a surface tension reduction. At high concentrations of surfactants, dissolved pollutants in the aqueous phase gain more mobility, which is conducive to removal and uptake by plants and even degradation by microbes. Also, the properties of the soil, and the surfactant itself, influence the adsorption of a surfactant (Mao et al., 2015).
The interaction and combination of LAS and other contaminants like MCs and metal ions has been found to be both synergistic and in some cases antagonistic (Chai et al., 2020). Our findings did not suggest any synergistic nor antagonistic effects of LAS in combination with metals and other contaminants such as MCs which were detected in the water used. Consistent with our findings, Zhang et al. (2008) did not find increased uptake of Cd by soybean in the presence of LAS. Jensen and Sverdrup (2002) also did not find any combined effect of LAS and pyrene on Folsomia fimetaria. According to Chai et al. (2020), synergistic or combined effects are influenced by a number of factors, including the types of contaminants tested, plant species, concentrations tested and the duration of exposure. In this study, factors such as faster biodegradation of LAS by microbes, a reduction in the exchangeable metals available in the media and low concentrations of LAS tested could all have affected LAS, metals and other contaminants' activity and toxicity to the plants.
What we also observed was a noticeably higher uptake in the edible parts of B. oleracea plants (leaves) compared to S. tuberosum plants (tubers). According to Hasan et al. (2019), B. oleracea, in the Brassicaceae family, belongs to a group of plants known to be hyperaccumulators and suited to grow in soils polluted with metals. Hyperaccumulators can take up Zn and Mn at concentrations up to 10 000 mg-kg-1; Cu, Ni and Pb beyond 1 000 mg-kg-1 dry mass, Cd at up to 100 mg-kg-1 dry mass in contaminated media (Hasan et al., 2019).
CONCLUSIONS AND RECOMMENDATIONS
This study explored the effect of an anionic surfactant (LAS in the form of SDS) on the accumulation of the metals aluminum (Al), manganese (Mn) and strontium (Sr) in Brassica oleracea (cabbage) and Solanum tuberosum (potato) plants when exposed to environmentally realistic concentrations of the pollutants. The findings indicated that when common cabbage (B. oleracea) and cultivated potato (S. tuberosum) plants were exposed to environmentally relevant concentrations of SDS and metals (Sr, Mn, Al and other cations), no negative effects could be observed on the plants. Moreover, the combined exposure of the plants to these contaminants did not result in increased uptake and accumulation of the metals as was anticipated. This implies that the existing levels of anionic surfactants such as LAS, metals and other contaminants such as MCs found in hyper-eutrophic reservoirs such as Roodeplaat and Hartbeespoort Dams in South Africa, pose little risk to the crop yields, quality of the crops and human health due to the possible accumulation of these contaminants in irrigated plants. Despite there being no immediate inherent risk to the plants and human health, continuous monitoring of the contaminants in water, soil and irrigated plants is recommended since the conditions, concentrations and other factors can quickly change if the management of the catchments does not improve in the near future.
CONFLICT OF INTEREST
None declared.
FUNDING
Funding for this study was granted by the South African Water Research Commission (WRC) Project No: K5/2972.
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
ORCIDS
Glynn K Pindihama: https://orcid.org/0000-0001-5537-7591
Mugera W Gitari: https://orcid.org/0000-0002-6387-0682
REFERENCES
ALMEIDA CMR, DIAS AC, MUCHA AP, BORDALO AA and VASCONCELOS MTSD (2009) Influence of surfactants on the Cu phytoremediation potential of a salt marsh plant. Chemosphere 75 (2) 135-140. https://doi.org/10.1016/jxhemosphere.2008.12.037 [ Links ]
AYERS RS and WESTCOT DW (1985) Water quality for agriculture. FAO Irrigation and Drainage Paper No. 29. FAO, Rome. [ Links ]
BASKAR V, VENKATESH J and PARK SW (2015) Impact of biologically synthesized silver nanoparticles on the growth and physiological responses in Brassica rapa ssp. pekinensis. Environ. Sci. Pollut. Res. 22 (22) 17672-17682. https://doi.org/10.1007/s11356-015-4864-1 [ Links ]
CHAI L, YANG L, ZHANG Y, ZHOU Y, WANG F and WU Z (2020) Antagonism or synergism? Responses of Hydrocharis dubia (Bl.) Backer to linear alkylbenzene sulfonate, naphthalene and their joint exposure. Ecotoxicol. Environ. Saf. 200 (January) 110747. https://doi.org/10.1016/j.ecoenv.2020.110747 [ Links ]
DWAF (Department of Water Affairs and Forestry, South Africa) (1996) Water Quality Guidelines Volume 4 Agricultural Use: Irrigation. Department of Water Affairs and Forestry, Pretoria. URL: http://www.dwa.gov.za/IWQS/wq_guide/edited/Pol_saWQguideFRESHIrrigationvol4.pdf (Accessed 11 May 2022). [ Links ]
FAO (2015) International Year of Soils 2015: Healthy soils for a healthy life. Rome, Italy. URL: https://www.fao.org/soils-2015/news/news-detail/en/c/277682/ (Accessed 30 March 2023). [ Links ]
FORNI C, BRAGLIA R, HARREN FJM and CRISTESCU SM (2012) Stress responses of duckweed (Lemna minor L.) and water velvet (Azolla filiculoides Lam.) to anionic surfactant sodium-dodecyl-sulphate (SDS). Aquat. Toxicol. 110-111 107-113. https://doi.org/10.1016/j.aquatox.2011.12.017 [ Links ]
HASAN MM, UDDIN MN, ARA-SHARMEEN I, ALHARBY HF, ALZAHRANI Y, HAKEEM KR and ZHANG L (2019) Assisting phytoremediation of heavy metals using chemical amendments. Plants 8 (9) 1-14. https://doi.org/10.3390/plants8090295 [ Links ]
HUANG L, LIU X, WANG Z, LIANG Z, WANG M, LIU M and SUAREZ DL (2017) Interactive effects of pH, EC and nitrogen on yields and nutrient absorption of rice (Oryza sativa L.). Agric. Water Manage. 194 (December) 48-57. https://doi.org/10.1016/j.agwat.2017.08.012 [ Links ]
JENSEN J and SVERDRUP LE (2002) Joint toxicity of linear alkylbenzene sulfonates and pyrene on Folsomia fimetaria. Ecotoxicol. Environ. Saf. 52 (1) 75-81. https://doi.org/10.1006/eesa.2002.2149 [ Links ]
LAWTON L, MARSALEK B, PADISÁK J and INGRID C (1999) Chapter 12. Determination of cyanobacteria In: Toxic Cyanobacteria in Water: A Guide to their Public Health Consequences, Monitoring and Management. E & FN Spon, London. 347-367. https://doi.org/10.1201/9781482295061-18 [ Links ]
MABASA HZ (2019) Spatial variability of aggregate stability, size distribution, erosion and runoff in selected soils in South Africa. Masters dissertation, University of Venda. URL: https://univendspace.univen.ac.za/bitstream/handle/11602/1647/Dissertation-Mabasa%2Ch.z.-.pdf?sequence=1&isAllowed=y [ Links ]
MACHADO J, CAMPOS A, VASCONCELOS V and FREITAS M (2017) Effects of microcystin-LR and cylindrospermopsin on plant-soil systems: A review of their relevance for agricultural plant quality and public health. Environ. Res. 153 (May 2016) 191-204. https://doi.org/10.1016/j.envres.2016.09.015 [ Links ]
MANSON AD and ROBERTS VG (2001) Analytical methods used by the Soil Fertility and Analytical Services Section. KZN Agri-Report No. N/A/2001/04 (4). KwaZulu-Natal Department of Agriculture and Rural Development. URL: https://www.kzndard.gov.za [ Links ]
MAO X, JIANG R, XIAO W and YU J (2015) Use of surfactants for the remediation of contaminated soils: A review. J. Hazardous Mater. 285 419-435. https://doi.org/10.1016/j.jhazmat.2014.12.009 [ Links ]
PANDEY P and GOPAL B (2010) Effect of detergents on the growth of two aquatic plants: Azolla pinnata and Hydrilla verticillata. Int. J. Sci. Technol. 5 (1) 107-114. [ Links ]
PIERATTINI EC, FRANCINI A, RAFFAELLI A and SEBASTIANI L (2018) Surfactant and heavy metal interaction in poplar: A focus on SDS and Zn uptake. Tree Physiol. 38 (1) 109-118. https://doi.org/10.1093/treephys/tpx155 [ Links ]
PINDIHAMA GK and GITARI MW (2020) Cyanobacterial toxins: an emerging threat in South African irrigation water. Water Environ. J. 34 (3) 506-516. https://doi.org/10.1111/wej.12473 [ Links ]
RASHID MH, FARDOUS Z, CHOWDHURY MAZ, ALAM MK, BARI ML, MONIRUZZAMAN M and GAN SH (2016) Determination of heavy metals in the soils of tea plantations and in fresh and processed tea leaves: An evaluation of six digestion methods. Chem. Centr. J. 10 (1) 1-13. https://doi.org/10.1186/s13065-016-0154-3 [ Links ]
SAQRANE S, GHAZALI I El, OUDRA B, BOUARAB L and VASCONCELOS V (2008) Effects of cyanobacteria producing microcystins on seed germination and seedling growth of several agricultural plants. J. Environ. Sci. Health - Part B Pesticides Food Contam. Agric. Wastes 43 (5) 443-451. https://doi.org/10.1080/03601230802062307 [ Links ]
SHAKYA K, CHETTRI MK and SAWIDIS T (2008) Impact of heavy metals (copper, zinc, and lead) on the chlorophyll content of some mosses. Arch. Environ. Contam. Toxicol. 54 (3) 412-421. https://doi.org/10.1007/s00244-007-9060-y [ Links ]
SULAIMAN FR and HAMZAH HA (2018) Heavy metals accumulation in suburban roadside plants of a tropical area (Jengka, Malaysia). Ecol. Process. 7 (1). https://doi.org/10.1186/s13717-018-0139-3 [ Links ]
VAN GINKEL CE (2004) A national survey of the incidence of cyanobacterial blooms and toxin production in major impoundments. Directorate: Resource Quality Services, Department of Water Affairs and Forestry, Pretoria. [ Links ]
VENKIDASAMY B, KARTHIKEYAN M and RAMALINGAM S (2019) Methods/protocols for determination of oxidative stress in crop plants. In: Hasanuzzaman M, Fotopoulos V, Nahar K and Fujita M (eds) Reactive Oxygen, Nitrogen and Sulfur Species in Plants: Production, Metabolism, Signaling and Defense Mechanisms. John Wiley & Sons, Hoboken. 421-435. https://doi.org/10.1002/9781119468677.ch17 [ Links ]
WANG Z, XIAO B, SONG L, WANG C and ZHANG J (2012) Responses and toxin bioaccumulation in duckweed (Lemna minor) under microcystin-LR, linear alkybenzene sulfonate and their joint stress. J. Hazardous Mater. 229-230 137-144. https://doi.org/10.1016/j.jhazmat.2012.05.109 [ Links ]
WANG Z, ZHANG J, SONG L, LI E, WANG X and XIAO B (2015) Effects of linear alkylbenzene sulfonate on the growth and toxin production of Microcystis aeruginosa isolated from Lake Dianchi. Environ. Sci. Pollut. Res. 22 (7) 5491-5499. https://doi.org/10.1007/s11356-014-3784-9 [ Links ]
ZHANG Y, LIAO B-H, ZENG Q-R, ZENG M and LEI M (2008) Surfactant linear alkylbenzene sulfonate effect on soil cd fractions and cd distribution in soybean plants in a pot experiment. Pedosphere. 18 (2) 242-247. https://doi.org/10.1016/s1002-0160(08)60013-2 [ Links ]
 Correspondence:
Correspondence:
Glynn K Pindihama
Email:gpindihama@gmail.com
Received: 30 July 2022
Accepted: 30 June 2023














