Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.49 n.2 Pretoria Apr. 2023
http://dx.doi.org/10.17159/wsa/2023.v49.i2.3919
RESEARCH PAPER
Water quality characteristics of Vanderkloof Dam and its potential for rainbow trout farming
KG SeanegoI; GC PitcherI, II; TA ProbynI; A Du RandtI; LM MansfieldI
IAquaculture Research, Department of Forestry, Fisheries and the Environment, Cape Town, South Africa
IIDepartment of Biological Sciences, University of Cape Town, Cape Town, South Africa
ABSTRACT
Vanderkloof Dam was periodically sampled between November 2014 and April 2016 for a range of water quality parameters to assess the potential for cage culture of rainbow trout, Oncorhyncus mykiss. The dam is strongly stratified in summer and autumn, although upper water column temperatures remain relativeh cool, largely <25°C. The dam is considered oligotrophic, characterised by phosphorus limitation of primarv production, particularly during summer. Light is strongly attenuated in the epilimnion and heterotrophic community metabolism is observed through much of the water column. Surface nutrients are depleted through the summer with phytoplankton assemblages dominated by the green algae, Oocystis lacustris, Desmodesmus bicaudatus and Coelastrum microporum. The water column turns over during the winter and the nearly isothermal impoundment approaches 11°C. Nutrients are re-introduced into surface waters following winter mixing coincident with an increase in diatoms. The preferred environmental window for rainbow trout becomes severely contracted during the summer in that epilimnion temperatures exceed 21°C and thermocline/hypolimnion oxygen concentrations fall below 3 mg-L-1.The coincidence of relatively high pH >9.5 could exacerbate these physiological challenges. Hydrological conditions for fish farming are most suitable in the proximity of the dam wall as opposed to further upstream. Here the hypoxic conditions that develop in the thermocline/hypolimnion during summer and autumn are less pronounced, particularly towards the southern bank, where concentrations of total suspended solids are generally <10 mg-L_1 and water clarity is more favourable for visual feeders such as fish. Although rainbow trout may survive the adverse conditions prevalent during the summer, growth is likely to be compromised and susceptibility to disease may increase.
Keywords: Vanderkloof Dam, water quality, phytoplankton, productivity, fish farming
INTRODUCTION
The Vanderkloof Dam (formerly PK Le Roux Dam) is situated approximately 130 km downstream of the Gariep Dam (originally Hendrik Verwoerd Dam) and is fed by the Orange River, South Africa's largest river. The dam was built in 1976 and is the second-largest storage reservoir in South Africa, with a capacity of about 3.2 χ 109 m3. The dam is important in the generation of hydroelectric power for peak and emergency situations and for providing irrigation water (DWS, 2014), and further serves as a popular recreational hub for angling and other water sports. The possibility of rainbow trout aquaculture in the dam has been previously investigated by the Rural Fisheries Programme, Rhodes University (unpublished report by Field and Rouhani). This assessment was based predominantly on water temperature as the key water quality parameter and is thus only preliminary in nature. A carrying capacity of 11 518 t-a-1 was calculated for the dam based on the projected release of phosphate by the cultured fish and allowable phosphate loading. Following on from this report, the commercial culture of rainbow trout in the dam was identified as a business opportunity by Operation Phakisa (DAFF, 2015).
The environmental variables of most importance to the growth and survival of fish are temperature, dissolved oxygen and food availability. Other factors such as pH, salinity, suspended solids, and unionised ammonia concentrations may be significant in certain circumstances. As this investigation is concerned with the aquaculture potential of artificially bred finfish stocked in cages and fed a formulated diet, considerations of food availability are not relevant.
Temperature is the most important abiotic factor controlling metabolism (Price et al., 1985). There are many abiotic and biotic factors that influence the temperature tolerances of fish in general, such as genetics/strain, temperature history, exposure duration, life stage, social stress and age. When conditions are outside of the optimal ranges, fish experience stress and slow growth, and under critical thermal limits the ability to swim is compromised (Jobling, 1981). There are also interactive effects between temperature and other environmental variables. The solubility of oxygen, for example, is reduced with increasing temperature.
Dissolved oxygen is another critically important environmental variable for consideration in assessing aquaculture potential. Conditions of reduced dissolved oxygen (hypoxia) and oxygen super-saturation (hyperoxia) are common occurrences in aquaculture and both can have marked effects on fish physiology and survival. In its simplest context, hypoxia in freshwater is most frequently defined as dissolved oxygen concentrations below 5-6 mg-L-1; a somewhat more conservative threshold than conventionally applied to the marine environment (Diaz et al., 2019). The application of an environmental concentration to describe hypoxia is subject to criticism as a level that is functionally hypoxic for a particular fish species will not apply to all species. Fish employ a number of different behavioural and physiological strategies that confer varying degrees of tolerance to reduced environmental oxygen (Vaquer-Sunyer and Duarte, 2008).
High concentrations of suspended solids have both direct and indirect effects on fish, thereby impacting habitat quality. Effects include gill trauma, adverse effects on swimming ability, altered blood chemistry, reduced resistance to disease, and impaired visual acuity due to increased turbidity (Bruton, 1985; Bash et al., 2001; Robertson et al., 2006). In extreme cases the ultimate outcome is reduced growth or even mortality. Generally the effect of suspended particles as stressors on fish is likely to be sub-lethal rather than lethal because of their ability to move away from high concentrations to areas of lower concentration should these be available (Kjelland et al., 2015). Guidelines for suspended solid concentrations have been proposed for freshwater fisheries by the European Inland Fisheries Advisory Commission (EIFAC 1964). In general TSS concentrations <25 mg-L-1 are unlikely to be harmful to fish or fisheries; concentrations of 25-80 mg-L-1 can support good to moderate fisheries, whereas concentrations >80 mg-L-1 are unlikely to support good fisheries.
Both low and high environmental pH can damage the outer surfaces of fish, including their gills, thereby affecting oxygen uptake, ionic balance and susceptibility to infection. In addition to the direct effects of pH on fish, environmental pH can influence the toxicity of other stressors. For instance, the equilibrium between the NH4+ ion and un-ionised NH3 is strongly pH and temperature dependent such that as they increase a higher proportion of total ammonia exists in the un-ionised form. As this is the toxic moiety a shift towards more alkaline waters will induce an increase in the toxic threat posed by ammonia.
The present study was undertaken to further investigate the water quality of Vanderkloof Dam with the intention of assessing its suitability for the aquaculture of rainbow trout. It supplements earlier studies on the dam with respect to its potential for fisheries (Allanson et al., 1983) and for trout aquaculture. The information contained herein contributes to providing a physical carrying capacity (PCC) of the dam as defined by McKindsey et al. (2006), in that the PCC depends largely on the physical and chemical attributes of the system as related to the requirements of the target species. This contrasts with the ecological carrying capacity (ECC) which primarily aims to minimise detrimental impacts on the environment (McKindsey et al., 2006). The development of a more conservative, management-orientated, ECC will require further study and mathematical modelling.
METHODS
Sampling
Seven field studies were undertaken in Vanderkloof Dam: 11-13 November 2014; 17-19 February 2015; 11-13 May 2015; 3-5 August 2015; 26-28 October 2015; 8-10 February 2016; and 25-27 April 2016. Dam water levels were approximately 10 m lower during February and April 2016 than during the other sampling periods. Starmon-mini temperature loggers were moored at a single station near the dam wall (Stn 1; Fig. 1) to provide continuous records of temperature at 4 depths (1.5 m, 8 m, 18 m and 51 m) from March 2015 to April 2016. Instrument profiles of the water column, and detailed process and water quality studies were undertaken at Stn 1 on each day of each field study. Discrete water samples were taken with a ΝΙΟ bottle from 7 depths (0, 5, 10, 15, 25, 50, 72 m) and stored on ice for further analyses. Instrument profiles were also conducted along two transects sampled during each field study; one along the main axis of the dam extending 44 km upstream and another across the dam near the wall (Fig. 1).
Water column profiles
Water column profiles of conductivity and temperature as related to depth were taken using a SBE-19Plus Seacat CTD. The Seacat was also equipped with additional sensors: a SBE 43 oxygen sensor, a WETLabs ECO fluorometer (470/695 nm) and turbidity sensor (optical scattering at 700 nm), a WETLabs C-Star transmissometer (660 nm), and a Biosperical QSP PAR (photo synthetically active radiation) irradiance sensor.
Turbidity and total suspended solids
Sediment in the water column was measured directly gravimetrically and indirectly as a component of turbidity and water clarity. Turbidity was measured using a WETLabs FLNTU fluorometer and expressed as nephelometric turbidity units (NTU). Dissolved coloured material also contributes to turbidity; however, the WETLabs instrument used in this study is unaffected by this component. Water clarity is a measure of visual distance through the water column and thus gives an indication of the distance a fish can see underwater. It is affected by both dissolved and particulate constituents. Beam transmittance (%) provided a measure of water clarity. Total suspended solids (TSS) provided the actual gravimetric measure of mineral and organic particles suspended in the water column (mg-L-1). Although these different measures are often correlated with each other, the strength of the correlations can vary markedly between systems and seasons. Choice of units will depend on whether the main concern is the physical mass of suspended sediment or its optical effects. Turbidity readings were converted to TSS (mg-L-1) using relationships established during each field study.
Suspended particulate matter was collected by filtration onto pre-combusted (450°C) 47 mm Whatman GF/F filters (nominal pore size 0.7 μm) that had been thoroughly rinsed with Milli-Q water under vacuum to remove friable material. Filters were weighed to the nearest 0.1 mg using a Radwag semi-micro, analytical balance. Generally, 1-2 L of sample was filtered, depending on particle load, and filters were stored in petri dishes at -20°C for later processing. On return to the laboratory the frozen filters were dried at 60°C, cooled in a desiccator, and reweighed for dry weight determinations. Filters were then fired at 450°C for measurement of percentage inorganic/organic solids.
Chlorophyll a, dissolved nutrients and pH
Chlorophyll a (Chi a), corrected for phaeopigments, was determined by fluorometry following extraction in 90%
acetone (Parsons et al., 1984). Samples for dissolved nutrient determinations were filtered (Whatman GF/F) immediately on collection. Shore-based analyses were generally initiated within 2 h of sampling, never longer than 4 h. Total ammonia nitrogen (NH4), soluble reactive phosphate (PO4) and nitrite (NO2) were measured in triplicate according to the manual methods described in Grasshoff et al. (1983), and nitrate (NO3) was determined following the cadmium reduction method of Nydahl (1976). All methods were scaled to 5 mL sample volume. Results are presented in μηιοί Ν or P -L_1 which can be converted to mg Ν or P by multiplication by 14/1 000 or 31/1 000, respectively.
Measurements of pH were conducted during three field studies using a Jenway 3510 pH meter
Productivity and phytoplankton
For determination of oxygen-based productivity and respiration rates samples, from 5 m and 15 m were incubated in situ for 24 h in large volume (10 L) polycarbonate carboys. Two 24 h incubations were undertaken during each field study. Changes in oxygen concentrations in the carboys were logged every 30 min by means of fast-response RINKO-1 optical sensors (Sasano et al., 2011). Daily net community production (NCP) was determined by the difference between initial and end-point oxygen concentrations. Hourly rates of respiration (R) were determined from declining oxygen concentrations at night. These were extrapolated to 24 h for daily respiration rates. Net community production (NCP) and respiration (R) as measured by changes in oxygen were converted to units of carbon (Odum, 1956; Engel et al., 2019).
Phytoplankton samples were fixed in borate-buffered formalin and enumerated following the Utermöhl method (Hasle, 1978). Algal community structure was quantified by classification analysis based on phytoplankton cell counts. The computer software package PRIMER 6 (Plymouth Routines in Multivariate Ecological Research) was used in the assessment of community structure using non-metric multidimensional scaling (Clarke and Gorley, 2006).
RESULTS
Temperature
The seasonal hydrography of the dam is clearly indicated by the continuous temperature recordings at 1.5, 8, 18 and 51 m depths at Stn 1 from March 2015 to March 2016 (Fig. 2). The epilimnion (upper layer) cools from late summer/autumn and the water column is entirely mixed by July, with temperatures continuing to fall. Stratification re-establishes in the spring as the epilimnion warms to temperatures in excess of 20°C in the upper layers in summer. There is a pronounced diurnal variation in surface water temperatures due to sun warming. The high variability at 18 m is most likely a result of adjustments in the depth of the thermocline. Temperatures in excess of 21°C occur in the upper epilimnion down to a depth of at least 8 m from December to March. Maximum temperatures (>25°C), occur intermittently at the surface in January and February. Deep waters show a gradual warming throughout the year except for the winter period of overturning when the almost isothermal water column cools to near 11°C. The cooler water at 18 m in 2016 reflects the shoaling of the thermocline owing to the reduced dam water levels.
The vertical temperature structure of the dam is consistent along its main axis (Fig. 3), with the intensification of stratification from spring to summer forming a distinct epilimnion and hypolimnion (lower layer) separated by a well-defined thermocline. The epilimnion was shallower during 2016, the consequence of reduced water levels in the dam. Autumn is characterized by a cooling of the epilimnion followed by complete mixing of the water column in winter.
Transverse sections of temperature show a similar seasonal pattern to that along the main axis, except that the thermocline deepens towards the banks of the dam, particularly the southern bank (Fig. 4).
Oxygen
The epilimnion remains well oxygenated throughout the year, though the thermocline and hypolimnion become hypoxic during the summer and autumn (Fig. 5). The oxycline is coincident with the thermocline, and duringthe summer of 2016 shoals upstream, indicating compression of the upper oxygenated layer.
Hypoxia develops initially in the thermocline in summer, subsequently spreading throughout the entire hypolimnion, Hypoxia is most intense upstream, away from the dam wall, with levels < 0.5 mg-L-1 throughout the hypolimnion in autumn, preceding the winter overturning. The water column is completely re-oxygenated during this period of winter mixing. Transverse sections reflect a similar pattern except that summer hypoxia is restricted to a narrow band in the thermocline (Fig. 6). There is also evidence for more favourable conditions towards the southern bank as the oxycline deepens.
Total suspended solids
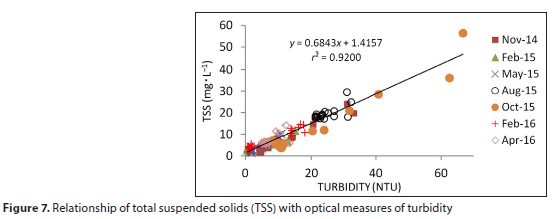
Concentrations of total suspended solids (TSS) were reasonably well correlated with optical measures of turbidity (NTU) and transmittance (%). The relationship departs from linearity at high TSS concentrations which is typical for these instruments. The overall relationship with turbidity for all sampling dates is shown in Fig. 7. However, conversions of turbidity to TSS were based on the individual relationship derived during each field study. The relationship with transmittance (not shown) was slightly weaker (TSS = 23.58 + 63.16 χ exp[-0.0095 χ transmittance], r2 = 0.9066).
Inorganic SS concentrations were well correlated with TSS on a dry-weight basis (Fig. 8). The relationship was such that the proportion of inorganic SS increased to a maximum of around 88% at 12 mg-L-1. Thus at concentrations above this level TSS was comprised largely of lithogenic material and thus of low organic content. A TSS concentration of around 3 mg-L-1 represents the transition from particles of primarily organic material to particles of primarily inorganic content.
The distribution of TSS along the main axis of the dam shows concentrations in the epilimnion increasing upstream but generally remaining below 25 mg-L-1 (Fig. 9). This concentration corresponds to a transmittance of 27% (from the equation given above). Concentrations are elevated in bottom waters, except during summer/autumn when particles accumulate mid-water, in the thermocline, particularly in the upper reaches of the dam, Maximum concentrations of 40-50 mg-L-1 were recorded during this period with slightly higher levels during 2016.
Transverse sections near the dam wall show a similar vertical distribution of TSS (Fig. 10). Epilimnion TSS concentrations remain low (< 10 mg-L-1) throughout the year except during winter mixing. A TSS of 10 mg-L-1 corresponds to a transmittance of 62%.
Chlorophyll a
Chlorophyll a concentrations (Chl a) along the main axis of the dam were mostly <4 μg·L-1 during spring. In summer, surface Chl a tended to exceed 10 μ gL-1 with upstream maxima of about 30 μg L-1 during February 2015, and 20 μg·L-1 during the dry February of 2016 (Fig. 11). In autumn, surface Chi a generally dropped to below 10 μgL-1 before declining further to below 4 μg L-1 in winter.
Transverse sections confirmed the lower biomass of waters in the proximity of the dam wall (Fig. 12). Here Chi a seldom exceeded 10 μg L-1.
Depth-integrated Chi a (mg-m-2) shows a pronounced seasonal pattern of maximum levels during summer/autumn and minima during winter/spring (Fig. 13). In 2015 there was a 4-fold difference between the low in spring and the high in autumn.
PH
Upper water column values exceeded pH 9 but remained below pH 9.5 (Fig. 14). The higher pH values, indicative of elevated photosynthesis, were measured during summer, coincident with the period of higher phytoplankton biomass (Fig. 11).
Nutrients
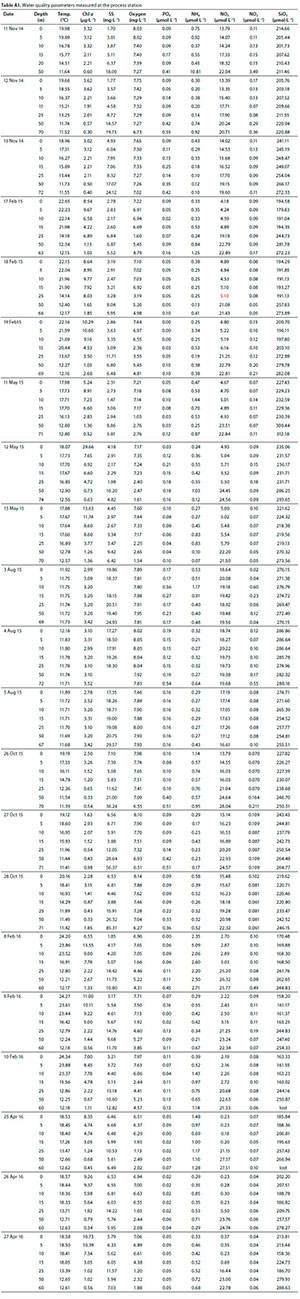
All nutrient data as measured at Stn 1 are presented in Table A1 (Appendix). Depth-integrated nutrients at Stn 1 were lowest during the summer/autumn period of peak Chi a and highest following winter mixing (Fig. 15). The system appears to be characterised by a marked lack of P with inorganic P04 concentrations 2 and 3 orders of magnitude lower than inorganic Ν and SiO3, respectively.
Primary productivity
Light was shown to be strongly attenuated in the surface waters of the dam with diffuse attenuation coefficients (Kd) for photosynthetic active radiation (PAR) ranging from 0.46 -2.05 m-1. PAR values were therefore low at 5 m (ranging from 0-155 mmol m-2-s-1) declining to below 1 mmol m-2-s_1 at 15 m depth (Fig. 16). As a result, GCP was notably higher at 5 m depth (20-177 mg C m day-1) compared to values at 15 m (10-61 mg C m-3-day-1), but no discernible seasonal trend was evident in GCP. Characteristically, R was a significant fraction of GCP at both depths and heterotrophic community metabolism (where NCP < 0) was therefore regularly observed in both surface (5 m) and deeper (15 m) waters.
Phytoplankton assemblages
Micrographs of the dominant phytoplankton species in Vander-kloof Dam are presented in Fig. 17. A total of 35 different phytoplankton taxa were recorded of which 54% were green algae, 20% diatoms, 9% dinoflagellates, and 8% euglenoids; cyanobacteria, cryptophytes and unidentified taxa each contributed 3% (Fig. 18a). Phytoplankton cell concentrations were notably higher in summer, specifically during February 2015, when cell concentrations exceeded 4 χ 105 cells-L-1 (Fig. 18b). Green algae were particularly prominent in summer at which time the species Oocystis lacustris, Desmodesmus bicaudatus and Coelastrum microporum were abundant. Diatoms dominated the assemblages of winter (August 2015) and spring (October 2015), exceeding concentrations of 1 χ 10; cells-L-1. These diatom assemblages were dominated by the species Aulacoseira granulata. Dinoflagellates generally made important contributions to phytoplankton assemblages, except during the above periods of diatom dominance, at which time dinoflagellates were absent from the plankton.
Non-metric multidimensional scaling analysis was used to group phytoplankton samples based on phytoplankton species abundances (Fig. 19). Ordination of samples by multidimensional scaling allowed the mapping or configuration of samples, in two dimensions. Community analyses were linked to the physical chemical and biological environment by superimposing values of temperature, nitrate and Chi a on the ordination plots. There is a general continuum in phytoplankton community composition from those assemblages dominated by diatoms in the cool, high-nitrate, low-biomass waters of winter (August 2015) and early spring (October 2015), to the mixed assemblages of late spring (November 2014) and late autumn (May 2015) characterised by intermediate temperatures, Chi a and nutrient concentrations, to the green algae dominated assemblages of summer (February 2015,2016) and mid-autumn (April 2016) characterised by higher temperatures, lower nutrients and higher biomass.
DISCUSSION
General characteristics of the dam
A study undertaken in 1977 by Allanson et al. (1983) to assess the fisheries potential of Vanderkloof Dam provides a useful comparison to the findings of the present study. The dam experiences one mixing event over an annual cycle (monomictic) during autumn/ winter, rendering it largely isothermal. For the remainder of the year the dam is highly stratified with a distinct epilimnion and hypolimnion separated by a well-defined thermocline. The dam is considered to be characterised by relatively low water temperatures in the summer, which were ascribed by Allanson et al. (1983) to a high evaporation rate as a result of low relative humidity and persistent winds. High concentrations of suspensoids were further considered by Allanson et al. (1983) to restrict the penetration of incident radiation, thus limiting the depth of sun warming to the uppermost 1.5 m. However, the results of the present study show diurnal fluctuations in temperature down to 8 m (Fig. 4), thereby contradicting this claim.
Allanson et al. (1983) found that dissolved oxygen concentrations in the hypolimnion were generally only 3 mg-L-1 lower than surface values of around 8.5 mg-L-1. The present study shows considerably greater differences between oxygen concentrations in the epilimnion and thermocline/hypolimnion (Fig. 5). These findings may be indicative of a long-term decline in oxygen in the reservoir since its construction.
TSS concentrations measured during the present study also appear to be lower (Fig. 10) than those reported by Allanson et al. (1983), although both studies report noticeably reduced values downstream (Fig. 7). The resulting reduction in turbidity was most evident near the dam wall where Allanson et al. (1983) reported a mean concentration of TSS of 37 mg-L-1 in surface waters whereas the current study reports TSS maxima in winter of <20 mg-L-1. Suspended matter in South African inland waters, including man-made dams, is largely derived from soil erosion of inorganic material (Walmsley and Bruwer, 1980). This predominance of mineral material, particularly at high turbidities, is clearly evident in the present TSS data (Fig. 8).
The mean summer Chi a of 7.47 μg·L-1 in the epilimnion is characteristic of mesotrophic waters (Nürnberg, 1996). However, mean estimates of NCP at both 5 m and 15 m depth were <0, indicating net heterotrophy. Attenuation coefficients were generally high and increased with increasing TSS concentrations. In addition to nutrient availability, high attenuation of light in the upper water column is therefore very likely to limit productivity in Vanderkloof Dam. Using a bioassay Selkirk (1982) similarly concluded that rapid light attenuation in the upper 1 m of the dam was the primary factor limiting algal growth. Heterotrophic community metabolism (where NCP < 0), as regularly observed in both surface (5 m) and deeper (15 m) waters, was generally driven by high rates of pelagic respiration. These high rates of respiration are likely fuelled by microbial respiration of allochthonous organic carbon from terrestrial sources (Ask et al., 2012). These microbial communities are also known to outcompete phytoplankton for nutrients, particularly phosphorus, and thereby contribute significantly to the very low phosphate levels.
Average PO4 concentrations in the epilimnion near the dam wall were low, ranging from 0.02 μmoΙ L-1 in autumn/summer to 0.18 μmol L-1 in the winter (Table A1, Appendix). In certain instances, PO4 concentrations were undetectable and it was only during winter mixing that concentrations exceeded 0.1 μmol L-1 in the epilimnion. The annual mean P04 concentration was 0.09 μmol L-1, which translates to 8.29 μg total P L-1, assuming PO4 is a third of total P (as shown by Allanson et al., 1983). Using 10 μg PT as the transition level from oligotrophy to eutrophy (Nürnberg, 1996) categorises the dam as oligotrophic with respect to nutrients, in accordance with Allanson et al. (1983). Additionally, both the soft-water geochemistry and relatively deep morphometry (mean depth 23 m) are typical of oligotrophic lakes (Nürnberg, 1996). The P04 concentrations reported in this study are lower than those reported by Allanson et al. (1983). They further reported seasonal stability in NO, and P04 which was interpreted as evidence for a lack of limitation of productivity by nutrients. This contrasts with the present results that show pronounced seasonal trends in nutrient concentrations (Fig. 14). Even though other sources of P can be used by phytoplankton, the decline in P04 to undetectable levels in summer when irradiance is maximal, suggests that P is very likely to limit productivity, particularly over this period. Allan-son et al. (1983) showed that there was little variation in nutrients along the length of the dam, suggesting the patterns shown at Stn 1 (Fig. 14, Table A1) may be indicative of the dam as a whole.
There is also evidence of a seasonal succession in phytoplankton species composition linked to seasonal trends in environmental conditions (Fig. 19). The dominance of green algae during summer months and the increase in diatoms during the winter turnover period, is characteristic of many oligotrophic African lakes (Evans, 1997; Darchambeau et al., 2014). These lakes typically exhibit net heterotrophy and have an R:GCP of > 1 (Ostrom et al., 2005).
Implications for rainbow trout farming
Vanderkloof temperatures from April to November, of 11 to 21°C, favour the culture of cold-water species such as rainbow trout. Even though trout can exhibit a high critical thermal maximum (Beitinger et al., 2000), growth rates and condition are impacted above 20°C. Several studies indicate that 21°C sets the upper limit in the vertical distribution of rainbow trout in lakes (Rowe and Chisnall, 1995). As a consequence, environmental temperatures exceeding the upper incipient lethal temperature of around 25-26°C (Hokanson et al., 1977; Raleigh et al., 1984; Bear et al, 2007) for protracted periods are not suitable for rainbow trout. This limit represents the theoretical threshold value below which 50% of the population can survive (Jobling, 1981).
There is no single oxygen concentration that defines tolerance in fish, as an increase in temperature causes metabolism to rise, while supply decreases with a fall in oxygen solubility. The massive depletion of oxygen in the thermocline and hypolimnion of the Vanderkloof Dam during autumn and summer (Fig. 5) is also of concern given the coincident rise in epilimnion temperatures. During the summer the 3 mg-L-1 oxygen isoline shoaled to 15.9 m in 2015 and 11.4 m in 2016, depth horizons that almost match the 21°C isotherm. Oxygen concentrations lethal to freshwater fish within 24 h are generally well below 3 mg-L-1, though salmonids appear to be more susceptible to reduced oxygen (Doudoroff and Shumway, 1970). The incipient lethal level of dissolved oxygen for adult and juvenile rainbow trout is about 3 mg-L-1, depending on other environmental conditions, in particular temperature (Raleigh et al., 1984). Given adequate supply of oxygen, rainbow trout have been shown to prefer temperatures around 16°C (Schurmann et al., 1991). Under hypoxia, cooler temperatures (12.7°C) are preferred Clearly, temperature and oxygen in the Vanderkloof Dam act in concert to cause a contraction of the water depth range most suitable for rainbow trout. Rowe and Chisnall (1995) described this phenomenon of habitat squeeze where their upper depth limit was defined by the 21°C isotherm and the lower limit by the 2.5 mg-L-1 oxygen isoline. Any compression of fish into a narrower depth stratum could have major implications for fish production and management, particularly in a culture scenario in which fish are held at greater than natural densities. In Vanderkloof Dam, habitat squeeze is less noticeable towards the dam wall and the hypoxic depth limit is also less pronounced toward the southern bank (Fig. 6). The strong hypoxic boundary that establishes during the autumn occurs when the upper column is more favourable from a temperature perspective; thus the fish have access to the well-oxygenated epilimnion without incurring temperature stress.
The downstream decrease in TSS (Fig. 8) similarly indicates more favourable conditions towards the dam wall. Although TSS concentrations are unlikely to constitute a serious stressor one also needs to consider the nature of the particles. Particle composition (inorganic versus organic) and size have significant effects on light attenuation properties, as shown by the greater mass-specific attenuation by inorganic particles than organic particles at small sizes (Smith and Davis-Colley, 2001). The inverse applies to larger particles. These, often overlooked, effects have potentially important implications for visual feeders such as fish. Berli et al. (2014) found that swimming ability of O. mykiss was reduced at moderate turbidity of 29 NTU (9.67 mg-L-1), a considerably lower turbidity than required for a similar response in brown trout, suggesting a greater sensitivity in rainbow trout. A literature review of the effects of suspended solids on salmonids by Newcombe and MacDonald (1991) shows that rainbow trout growth can be reduced at TSS concentrations of 50-84 mg-L-1 over periods of 2 weeks or longer. For rainbow trout culture the TSS concentrations of <20 mg-L-1 in the Vanderkloof Dam are unlikely to impact negatively.
Both low and high environmental pH can damage the outer surfaces of fish such as the gills and the skin thereby affecting oxygen uptake, ionic balance and susceptibility to infection. The recommended range for rainbow trout is pH 6-9 (Hartmann and Miles, 2001); however, growth is optimized in slightly alkaline waters of pH 7-8 (Swales, 2006). Swimming behaviour has been shown to be unaltered between pH 6 and 9 (Ye and Randall, 1991). For most fish species, short-term exposures to high pH (pH ~ 9.5) are rarely lethal. Besides damaging outer surfaces such as the gills, eyes and skin, high pHs can affect the olfactory system of fish, inhibiting their ability to respond to certain chemicals (Hara, 1976). Olfactory cues might assume more importance to a visual feeder in turbid systems as visibility deteriorates. Thus, the relatively high pH (pH > 9) in the epilimnion of Vanderkloof Dam in summer is of some concern. The potential lethal effects of high pH can be ameliorated by high Ca++ concentrations (McDonald et al., 1980; Yesaki and Iwama, 1992). However, Vanderkloof Dam water is soft, containing only low concentrations of Ca++ (Allanson et al., 1983). In addition, during these summer conditions of high pH and temperature more than 40% of total ammonia (NH4) exists in the toxic un-ionised form. Natural concentrations of NH4 in the dam (Table A1, Appendix) pose no threat to the wellbeing of fish. However, excreted NH4 could accumulate to levels of concern, particularly if the fish are crowded into a confined water column space as a result of habitat compression.
Vanderkloof Dam has notably low levels of cyanobacteria, which often occur in warm water where nitrogen is limited (Du Plessis et al., 2007; Venter et al., 2000). Dominance of green algae has been recorded at temperatures between 21 and 23°C in Lake Chivera, Zimbabwe, while cyanobacteria dominated at 25-26°C (Ndebele-Murisa et al., 2010). Thus, the low number of cyanobacteria in Vanderkloof may be attributed to the relatively coolertemperatures of the dam. Cyanobacteria, particularly Microcystis spp. that produce toxins, are toxic to both animals and humans, with some species producing taste and odour compounds that affect the palatability of fish (Harding and Paxton, 2001). Nutrient sources, including organic waste from aquaculture activities, may favour the proliferation of phytoplankton, and Du Plessis et al. (2007) have reported a dominance of cyanobacteria as a consequence of rainbow trout culture in irrigation dams in the Western Cape Province, South Africa. These observations highlight the need for regular monitoring of changes in plankton community.
While the temperature conditions are sub-optimal for rainbow trout outside of winter, the prospects of culturing other species are less. For example, high growth rates of Mozambique tilapia and sharptooth catfish are achieved at temperatures of 25-30°C and 25-33°C, respectively (Price et al., 1985; Britz and Hecht, 1987) -temperatures notably higher than those reported for Vanderkloof Dam. Vanderkloof Dam temperatures are therefore likely to restrict the growth of these species irrespective of the season. In the Gariep Dam, which is 130 km upstream of the Vanderfloof Dam, favourable temperatures of >22°C (but not optimal) for culturing common carp and sharptooth catfish are limited to only 110 days of the year (Van Niekerk and Moloi, 2018). Generally low abundance of banded tilapia, sharptooth catfish and largemouth yellowfish in Vanderkloof Dam as a consequence of these lower temperatures has been reported by Allanson et al. (1983), further emphasising thermal constraints. Smallmouth yellowfish are more adaptable to conditions in the dam, but they are not viable for commercial production as they are slow growing (O'Brien and De Villiers, 2011).
The potential ecological implications that may arise if rainbow trout were to escape in the Vanderkloof Dam, such as predator displacement, changes in biodiversity, hybridization and diseases, have been assessed through an ecological risk assessment (DAFF, 2017). The report indicated that, while they have the ability to compete with predatory fish in the Vanderkloof Dam such as largemouth yellowfish, their survival and growth would be severely limited. More specifically, summer temperatures which can reach lethal limits are not conducive for the establishment of a viable feral population.
Notwithstanding the preceding discussion on potential stressors, many of the experimental findings on fish tolerances to key water quality parameters may not mimic the situation under culture. The resilience of O.mykiss to the less than ideal summer conditions in Vanderkloof Dam can only realistically be assessed through pilot-scale ventures. A pilot-scale trout facility in the dam is subject to compliance with the National Environmental Management: Biodiversity Act 2004 (Act No. 10 of 2004) (RSA, 2020). Best management practices would therefore have to be employed and monitoring efforts would need to be put in place to ensure environmental sustainability.
ACKNOWLEDGEMENTS
Thank you to Dr Archiebold Hlungwani for producing the map in Fig. 1 and Dr Sanet Janse Van Vuuren for assisting with the phytoplankton identification.
AUTHOR CONTRIBUTIONS
KG Seanego was responsible for analytical nutrient analysis, measuring pH, phytoplankton identification and enumeration. Additionally contributed to data analysis and writing of the paper.
Dr GC Pitcher was responsible for the conceptualisation and methodology of the project, processing of incubation samples, provided comments and suggestions and edited final version of the paper.
Dr TA Probyn, was responsible for the conceptualisation and methodology of the project and retrieval of data from instruments and analysis thereof. Initiated the first draft of the paper.
Mr A Du Randt and Ms L Mansfield carried out field sample collection and instrument deployment and retrieval. Ms Mansfield took photgraphs of live phytoplankton.
ORCIDS
KG Seanego: https://orcid.org/0000-0002-1540-0416
GC Pitcher: https://orcid.org/0000-0001-8536-9314
REFERENCES
ALLANSON BR, BEUTHIN CL, JANSEN CJ and SELKIRK WT (1983) The physical and chemical limnology of Lake Ie Roux. In: Allanson BR and Jackson PBN (eds) Limnology and fisheries potential of Lake Ie Roux. South African National Scientific Programmes Report 77 4-25. [ Links ]
ASK J, KARLSSON J and JANSSON M (2012) Net ecosystem production in clear-water and brown-water lakes. Global Biogeochem. Cycles 26 1017. https://doi.org/10.1029/2010GB003951 [ Links ]
BASH J, BERMAN C and BOLTON S (2001) Effects of turbidity and suspended solids on salmonids. Washington State Transportation Commission and US Department of Transportation. 92 pp. [ Links ]
BEAR EA, MCMAHON TE and VALE AV (2007) Comparative thermal requirements of westslope cutthroat trout and rainbow trout: Implications for species Interactions and development of thermal protection standards. Trans. Am. Fish. Soc. 136 1113-1121. https://doi.org/10.1577/T06-072.1 [ Links ]
BEITINGER TL, BENNETT WA and MCCAULEY RW (2000) Temperature tolerances of North American freshwater fishes exposed to dynamic changes in temperature. Environ. Biol. Fish. 58 237-275. https://doi.Org/10.1023/a:1007676325825 [ Links ]
BERLI BI, GILBERT MJH, RALPH AL, TIERNEY KB and BURKHARDT-HOLM P (2014) Acute exposure to a common suspended sediment affects the swimming performance and physiology of juvenile salmonids. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 176 1-10. https://doi.org/10.1016/jxbpa.2014.03.013 [ Links ]
BRITZ PJ and HECHT T (1987) Temperature preferences and optimum temperature for growth of African sharptooth catfish (Clariasgriepinus) larvae and post-larvae. Aquaculture 63 205-214. https://doi.org/10.1016/0044-8486(87)90072-X [ Links ]
BRUTON MN (1985) The effects of suspensoids on fish. In: Davies BR and Walmsley RD (eds) Perspectives in Southern Hemisphere Limnology. Springer, Dordrecht. 221-241. https://doi.org/10.1007/978-94-009-5522-6_16 [ Links ]
CLARKE KR and GORLEY RN (2006) PRIMER V6: User Manual/Tutorial. PRIMER-E, Plymouth. 192 pp. [ Links ]
DAFF (Department of Agriculture, Forestry and Fisheries, South Africa) (2015) Operation Phakisa: Unlocking the Economic Potential of South Africa's Oceans. Aquaculture Year One Review Oct 2014 - Oct 2015. Department of Agriculture, Forestry and Fisheries, Pretoria. 29 pp. [ Links ]
DAFF (Department of Agriculture, Forestry and Fisheries, South Africa) (2017) Ecological Risk Assessment: For the grow out of Rainbow Trout (Oncorhynchus mykiss) in the Vanderkloof Dam. Compiled by Ecosense CC. Department of Agriculture, Forestry & Fisheries, Pretoria. 83 pp. [ Links ]
DARCHAMBEAU F, SARMENTO H and DESCY JP (2014) Primary production in a tropical large lake: The role of phytoplankton composition. Sei. Total Environ. 473 178-188. https://doi.org/10.1016/j.scitotenv.2013.12.036 [ Links ]
DIAZ RJ, ROSENBERG R and STURDIVANT K (2019) Hypoxia in estuaries and semi-enclosed seas. In: Laffoley D and Baxter JM (eds) Ocean Deoxygenation: Everyone's Problem - Causes, Impacts, Consequences and Solutions. IUCN, Gland. 85-102. [ Links ]
DOUDOROFF P and SHUMWAY DL (1970) Dissolved oxygen requirements of freshwater fishes. FAO Technical Paper 86, 291. Food and Agriculture Organization of the United Nations, Geneva. [ Links ]
DU PLESSIS D (2007) Impacts of cage aquaculture on the farm dam ecosystem and its use as a multipurpose resource: Implications for irrigation. MSc thesis, University of Stellenbosch. [ Links ]
DWS (Department of Water and Sanitation, South Africa) (2014) Vanderkloof Dam final resource management plan. Report compiled by Nemai Consulting. Department of Water and Sanitation, Pretoria. 86 pp. [ Links ]
EIFAC (European Inland Fisheries Advisory Commission) (1964) Water quality criteria for European freshwater fish. Report on finely divided solids and inland fisheries. EIFAC Technical Paper No. 1. EIFAC Working Party on Water Quality Criteria for European Freshwater Fish. European Inland Fisheries Advisory Commission, FAO, Rome. [ Links ]
ENGEL F, ATTERMEYER K, AYALA AI, FISCHER H, KIRCHESCH V, PIERSON DC and WEYHENMEYER GA (2019) Phytoplankton gross primary production increases along cascading impoundments in a temperate, low-discharge river: Insights from high frequency water quality monitoring. Sei. Rep. 9 (1) 1-13. https://doi.org/10.1038/S41598-019-43008-W [ Links ]
EVANS JH (1997) Spatial and seasonal distribution of phytoplankton in an African Rift Valley lake (L. Albert, Uganda, Zaire). Hydrobiologia. 354 1-16. https://doi.Org/10.1023/A:1003026415788 [ Links ]
FIELD R and ROUHANI Q (unpublished) A preliminary investigation to the potential cage-culture of rainbow trout in Vanderkloof Dam in the Northern Cape Province. Report compiled by the Rural Fisheries Programme, Rhodes University. Department of Agriculture, Land Reform and Rural Development Northern Cape Province. 32 pp. [ Links ]
GRASSHOFF P (1983) Methods of seawater analysis. Verl. Chem. 419 61-72. [ Links ]
HARA TJ (1976) Effects of pH on the olfactory responses to amino acids in rainbow trout, Salmo gairdneri. Comp. Biochem. Physiol A. Comp. Physiol. 54 37-39. https://doi.org/10.1016/s0300-9629(76)80068-0 [ Links ]
HARDING WR and PAXTON BR (2001) Cyanobacteria in South Africa: a review. WRC Report No.TT 153/01. Water Research Commission, Pretoria. 172 pp. [ Links ]
HARTMAN G and MILES M (2001) Assessment of techniques for rainbow trout transplanting and habitat management in British Columbia. Canadian Manuscript Report of Fisheries and Aquatic Sciences 2562. Habitat Enhancement Branch, Fisheries and Oceans Canada, Vancouver. [ Links ]
HASLE GR (1978) The Inverted Microscope Method. Phytoplankton Manual. UNESCO, Paris. 88-96. [ Links ]
HOKANSON KEF, KLEINER CF and THORSLUND TW (1977) Effects of constant temperatures and diel fluctuations on specific growth and mortality rates and yield of juvenile rainbow trout, Salmo gairdneri. Fish. Res. Board Can. 34 639-648. https://doi.org/10.1139/177-100 [ Links ]
IOBLING M (1981) Temperature tolerance and the final preferendum-rapid methods for the assessment of optimum growth temperatures. ƒ. Exp. Biol. 19 439-455. https://doi.org/10.1111/j.1095-8649.1981.tb05847.x [ Links ]
KJELLAND ME, WOODLEY CM, SWANNACK TM and SMITH DL (2015) A review of the potential effects of suspended sediment on fishes: potential dredging-related physiological, behavioral, and transgenerational implications. Environ. Syst. Decis. 35 (3) 334-350. https://doi.org/10.1007/sl0669-015-9557-2 [ Links ]
MCDONALD DG, HOBE H and WOOD CM (1980) The influence of calcium on the physiological responses of the rainbow trout, Salmo gairdneri, to low environmental pH. J. Exp. Biol. 88 (1) 109-132. https://doi.org/10.1242/jeb.88.L109 [ Links ]
MCKINDSEY CW, THETMEYER H, LANDRY T and SILVERT W (2006) Review of recent carrying capacity models for bivalve culture and recommendations for research and management. Aquaculture 261 (2) 451-462. https://doi.Org/10.1016/j.aquaculture.2006.06.044 [ Links ]
NDEBELE-MURISA MR, MUSH C and RAITT L (2010) A review of phytoplankton dynamics in tropical African lakes. S. Afr. J. Sei. 106 13-18. https://doi.org/10.4102/sajs.v106il/2.64 [ Links ]
NEWCOMBE CP and MACDONALD DD (1991) Effects of suspended sediments on aquatic ecosystems. N. Am. Fish. Manage. 11 72-82. https://doi.org/10.1577/1548-8675(1991)01K0072:EOSSOA>2.3.CO;2 [ Links ]
NÜRNBERG GK (1996) Trophic state of clear and coloured, soft- and hard water lakes with special consideration of nutrients, anoxia, phytoplankton and fish. Lake Reserv. Manage. 12 432-447. https://doi.org/10.1080/07438149609354283 [ Links ]
NYDAHL F (1976) On the optimum conditions for the reduction of nitrate to nitrite by cadmium, lalanta 23 (5) 349-357. https://doi.org/10.1016/0039-9140(76)80047-1 [ Links ]
O'BRIEN G and VILLIERS P (2011) Biology and ecology of the Orange-Vaal largemouth and smallmouth yellowfishes in the Vaal River. WRC Report No. TT 508/11. Water Research Commission, Pretoria. [ Links ]
ODUM HT (1956) Primary production in flowing waters 1. Limnol. Oceanogr. 1 (2) 102-117. https://doi.org/10.4319/lo.1956.L2.0102 [ Links ]
OSTROM NE, RUSS ME, FIELD A, PIWINSKI L, TWISS MR and CARRICK HJ (2005) Ratios of community respiration to photosynthesis and rates of primary production in Lake Erie via oxygen isotope techniques, J. Great Lakes Res. 31 (2) 138-153. https://doi.org/10.1016/s0380-1330(05)70310-5 [ Links ]
PARSONS TR, MAITA Y and LALLI CM (1984) A Manual of Chemical and Biological Methods for Seawater Analysis. Pergamon Press, Oxford. 173 pp. [ Links ]
PRICE EE, STAUFFER JR and SWIFT MC (1985) Effect of temperature on growth of juvenile Oreochromis mossambicus and Sarotherodon melanotheron. Environ. Biol. Fishes. 13 (2) 149-152. https://doi.org/10.1007/BF00002583 [ Links ]
RALEIGH RF, HICKMAN T, SOLOMON RC and NELSON PC (1984) Habitat suitability information: Rainbow trout. FWS/OBS-82/10.60. United States Fish and Wildlife Service, Lafayette, La. 64 pp. [ Links ]
ROBERTSON MJ, SCRUTON DA, GREGORY RS and CLARKE KD (2006). Effect of suspended solids of freshwater fish and habitat. Can. Lech. Rep. Fish. Aquat. Sei. 2 644 pp. [ Links ]
ROWE DK and CHISNALL BL (1995) Effects of oxygen, temperature and light gradients on the vertical distribution of rainbow trout, Oncorhynchus mykiss, in two North Island, New Zealand, lakes differing in trophic status. N. Z. J. Mar. Freshwater Res. 29 (3) 421-434. https://doi.org/10.1080/00288330.1995.9516676 [ Links ]
RSA (Republic of South Africa) (2020) National Environmental Management: Biodiversity Act, 2004 (Act No. 10 of 2004). Alien and invasive species lists, 2020. Government Gazette no. 43726. 18 September 2020. URL: https://www.greengazette.co.za/notices/national-environmental-management-biodiversity-act-2004-act-no-10-of-2004-alien-and-invasive-species-lists-2020_20200918-GGN-43726-01003 [ Links ]
SASANO D, ISHII M, MIDORIKAWA T, NAKANO T, TOKIEDA T and UCHIDA H (2011) Testing a new quick response oxygen sensor, "RINKO." Meteorol. Geophys. 62 63-73. https://doi.org/10.2467/mripapers.62.63 [ Links ]
SCHURMANN H, STEFFENSEN JF and LOMHOLT JP (1991) The influence of hypoxia on the preferred temperature of rainbow trout Oncorhynchus mykiss. J. Exp. Biol. 157 75-86. https://doi.org/10.1242/jeb.l57.1.75 [ Links ]
SELKIRK WT (1982) An analysis, by bioassay, of the factors which limit algal growth in the PK Ie Roux impoundment, Orange River, South Africa. Hydrobiologia 97 (2) 151-156. https://doi.org/10.1007/bf00011968 [ Links ]
SMITH D and DAVIS-COLLEY R (2001) Turbidity suspended sediment and water clarity, J. Am. Water Resour. Assoc. 37 1085-1101. https://doi.org/10.1111/j.l752-1688.2001.tb03624.x [ Links ]
SWALES S (2006) A review of factors affecting the distribution and abundance of rainbow trout (Oncorhynchus mykiss Walbaum) in lake and reservoir systems. Lake Reserv. Manag. 22 (2) 167-178. https://doi.org/10.1080/07438140609353894 [ Links ]
VAN NIEKERK JA and MOLOI Z (2018) Introduction of extensive cage culture systems for breeding of catfish (Clarius Gariepinus) and common carp (Cyprinus Carpio) at the Aquaculture Technology Demonstration Centre, Xhariep District: An agricultural extension perspective. S. Afr. J. Agric. Ext. 46 (1) 106-112. https://doi.org/10.17159/2413-3221/2018/v46nla466 [ Links ]
VAQUER-SUNYER R and DUARTE CM (2008) Thresholds of hypoxia for marine biodiversity. Proc. Natl. Acad. Sci. USA 105 (40) 15452-15457. https://doi.org/10.1073/pnas.0803833105 [ Links ]
VENTER GC, ROOS JC and VAN WYK PWJ (2000) Water quality of the Upper Orange River. MSc thesis, University of the Orange Free State. [ Links ]
WALMSLEY RD and BRUWER CA (1980) Water transparency characteristics of South African impoundments. Limnol. Soc. South. Afr. 6 69-76. https://doi.org/10.1080/03779688.1980.9634548 [ Links ]
YE X and RANDALL DJ (1991) The effect of water pH on swimming performance in rainbow trout (Salmo gairdneri, Richardson). Fish Physiol. Biochem. 9 15-21. https://doi.org/10.1007/BF01987607 [ Links ]
YESAKI TY and IWAMA GK (1992) Some effects of water hardness on survival, acid-base regulation, ion regulation, and ammonia excretion in rainbow trout in highly alkaline water. Physiol. Zool. 65 (4) 763-787. https://doi.org/10.1086/physzool.65.4.30158538 [ Links ]
 Correspondence:
Correspondence:
Koena Seanego
Email:gseanego@gmail.com
Received: 19 July 2021
Accepted: 8 March 2023
APPENDIX