Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Water SA
On-line version ISSN 1816-7950
Print version ISSN 0378-4738
Water SA vol.49 n.1 Pretoria Jan. 2023
http://dx.doi.org/10.17159/wsa/2023.v49.i1.3988
RESEARCH PAPER
Cyclopia subternata growth, yield, proline and relative water content in response to water deficit stress
MS MahlareI; MN LewuII; FB LewuI; C BesterII
IDepartment of Agriculture, Faculty of Applied Sciences, Cape Peninsula University of Technology, Private Bag X8, Wellington 7654, South Africa
IIARC Infruitec-Nietvoorbij, Private Bag X5026, Stellenbosch 7599, South Africa
ABSTRACT
Cyclopia, generally known as honeybush, and belonging to the Fabaceae family, originates from the Cape Floristic Region of the Eastern Cape and Western Cape provinces of South Africa. Currently, 6 honeybush species are commercially cultivated but, to date, there have been limited trials attempting to study their agronomic water demand. A pot trial was conducted where Cyclopia subternata plants were cultivated on different soil types (Stellenbosch granite, Stellenbosch shale and Stellenbosch clovelly) and subjected to three different water-deficit stress levels (well-watered, semi-stressed and stressed). Remarkably, irrigation treatments and soil types did not significantly affect the growth of the plants. However, the well-watered treatment consistently had higher yields compared to the other two treatments. The water-stressed (semi-stressed and stressed) treatments had lower relative water contents (RWC) with higher concentrations of proline, which signify water stress, compared to the control treatment. Higher proline and lower RWC contents found in this study are indications of water stress.
Keywords: honeybush, proline content, relative water content, tea plant, water deficit stress
INTRODUCTION
South Africa, a drought-prone country, is home to rooibos (Aspalathus linearis), bush (Athrixia phylicoides) and honeybush (Cyclopia species) teas (Joubert et al., 2011). The teas are sold as either black or green (fermented or unfermented, respectively) (Horn, 2019). Even though the commercialization of some of these remedial teas is still in its infancy stage, honeybush has gained recognition, while rooibos is the most well-known and well established in the industry (Van Wyk and Gericke, 2000; Joubert et al., 2008; Joubert et al., 2011).
Studies state that these South African indigenous tea species have essential nutrients (iron, calcium, magnesium, copper, and potassium) that can improve wellbeing and/or prevent diseases, and have economic potential (Rampedi and Olivier 2005; McGaw et al., 2007). These herbal teas are famous for their rich caffeine-free and organic antioxidant properties, which are helpful in colon, throat and lung illnesses, prevention of urinary stone and tooth caries and other medical problems (Soni et al., 2015).
The demand for honeybush tea has prompted concerns of over-exploitation of natural populations of the Cyclopia species. The increased rate of wild harvesting diminishes the natural population, thus making the exploitation of Cyclopia species unsustainable. Harvesting practices have contributed to the decrease and even disappearance of populations of the wild Cyclopia species (Du Toit et al., 1998). Other factors threatening the growth of the honeybush industry include drought and veld fires. To ensure sustainable production, commercial honeybush plantations have been established (Joubert et al., 2011).
Commercial production is therefore becoming increasingly important to save the natural populations from decline while ensuring consistent supply. Cultivation of Cyclopia species will not only contribute to sustainability and conservation of the species but will also improve the livelihoods of rural harvester communities. Although cultivated honeybush plants receive water through irrigation in addition to rainfall, irrigation volume is at the discretion of farmers, without any understanding of the water requirements of the species. Presently, the shortage of water has massively increased in some parts of the world, including some regions in South Africa, due to a variety of reasons such as an ever-increasing population, industrialization, water pollution and poor management, climate change and others (WWAP, 2012; Connor, 2015; Long and Pijanowski, 2017).
In addition, the South African Department of Water and Sanitation (DWS) has reduced agricultural allocations significantly, and irrigation volume for the agricultural sector is unlikely to increase anytime soon. For example, in 2015, DWS restricted an irrigation water allocation in KwaZulu-Natal by 40-100% due to a water shortage caused by insufficient rain (RSA, 2015). Also, agriculture in the Western Cape has had to cut its water use by 60% since 2017 (WWF, 2018). As a result, research that focuses on the sustainability of water-use in agriculture is gaining huge interest (Velasco-Munoz et al., 2018). Environmental factors, including water stress, tend to interfere with crucial physiological processes and biochemical mechanisms; resulting in yield loss (Per et al., 2017).
Therefore, research on water-use and the effects of stress on plant growth is crucial for production sustainability in agriculture (Harb et al., 2010). Plants have proven to use protective mechanisms such as proline and carbohydrate accumulation to cope with water-deficit situations (Mabizela, 2020). Proline is a water-soluble amino acid and beneficial solute that accumulates in plants under different kinds of stresses, such as drought, cold, heat, heavy metal, nutrient, and salt stress (Siddique and Dubey, 2017).
Relative water content (RWC) is a useful measure of plant water status in terms of the physiological consequences of cellular water deficit and may indicate the degree of water stress expressed under drought and heat stress (Surendar et al., 2013; Soltys-Kalina et al., 2016). It combines leaf water potential and the effect of osmotic regulation to quantify plant water status (Lugojan and Ciulcas, 2011; Kardile et al., 2018). Insufficient water in plants due to stress results in low RWC (Chakhchar et al., 2015).
A plant's ability to retain turgor during water-deficit periods guarantees smooth metabolic processes for growth (Cerekovic et al., 2013). Several studies have stated that RWC determination is an efficient method of assessing drought tolerance and plant water status (Slabbert and Krüger, 2004; Li-Ping et al., 2006; Jones, 2007; Obidiegwu et al., 2015). To date, limited studies have been conducted to investigate the water needs of Cyclopia species. Therefore, the aim of this study was to evaluate the effects of 3 different irrigation treatments on growth, yield, proline and relative water content of Cyclopia subternata species of honeybush.
METHODS AND MATERIALS
Experimental site and layout
A greenhouse pot trial was conducted at the Agricultural Research Council (ARC), Infruitec-Nietvoorbij (latitude -33.914395° and longitude 18.861390°) in Stellenbosch, South Africa, to determine the effect of water stress on C. subternata. The experiment was conducted for 140 days (from end-July to mid-December 2020). The experimental design was a randomised block design (RBD) with 9 treatment combinations (irrigation x soil type) replicated at random in each of 4 block replicates. The treatment structure was a 3 x 3 factorial with 3 irrigation levels (well-watered, semi-stressed and stressed) and 3 soil types (Stellenbosch granite, Stellenbosch shale and Stellenbosch clovelly).
Soil collection, preparation, and planting
Soil collection was carried out from three different sites at the ARC Nietvoorbij research farm. For each site, soil samples were collected from the 0-30 cm soil depth, sieved with a 3 mm sieve to remove large fragments, followed by baseline physicochemical analysis of the composited samples at a commercial laboratory (Bemlab, Strand). 14 kg of soil was weighed into a 30 cm plastic pot, using a digital scale. The soil in each pot was irrigated to pot capacity (PC) before planting. Nine-month-old honeybush (C. subternata) seedlings were transplanted to one plant per pot. The plants were well-watered for 5 weeks to ensure good establishment before introducing the different irrigation treatments.
Irrigation and weed control
From the 6th week after transplanting (WAT), C. subternata plants were subjected to 3 different irrigation treatments for 105 days (September-December 2020). The well-watered treatment (control) received 500 mL of water 3 times a week, semi-stressed received the same quantity of water twice a week while the stressed treatment received 500 mL of water once a week until the end of the study. The plants were hand irrigated with an Erlenmeyer flask. Weeds that emerged in the pots during the trial period were mostly broad-leaved plants. The weeds were either hand-pulled or manually removed using a garden fork immediately after irrigation when the soil was still wet.
Data collection
Growth parameters
Measurement of growth parameters commenced at 6 WAT on a monthly basis, until the trial was terminated in December 2020. Plant height was measured from the soil surface to the tip of the longest shoot, using a tape measure, stem diameter was measured with a digital Vernier caliper while the stem circumference was calculated from stem diameter values using the following formula:

where π = 3.14 and d = diameter
Total yield (shoot and root biomass)
At the end of the study (20 WAT), the above-ground biomass (shoot) was cut just above the soil surface using a pruning shear, placed in a labelled paper bag and then weighed using a sensitive weighing balance to obtain the fresh mass of the shoot. The fresh shoot was oven-dried at 70°C for 24 h. The dried samples were also weighed and recorded using a sensitive digital scale to 4 decimal places. The root biomass was determined by washing plant roots from each pot under running water using a 0.053 mm sieve in order to separate the roots from the soil and prevent loss of fine roots. The washed roots were air-dried overnight and weighed using a sensitive scale. Total plant yield is the combined fresh weight of the above-ground biomass and the air-dried root biomass.
Estimation of proline content using the colorimetric method
Determination of the proline content of C. subternata commenced at 6 WAT using the modified method of Abraham et al. (2010). Leaf samples were collected at 2-week intervals during the growth period. The extraction procedure was done by crushing 0.1 g of fresh frozen leaves in 0.5 mL of 3% sulfosalicylic acid (w/v), using a plastic test tube and pestle. The homogenised extracts were centrifuged for 5 min at a speed of 13 500 r/min. A reaction mixture of 0.1 mL of 3% sulphosalicylic acid, 0.2 mL of glacial acetic acid, 0.2 mL of acid ninhydrin buffer (1.25 g ninhydrin, 30 mL glacial acetic acid, and 20 mL of 6 M phosphoric acid) and 0.1 mL of centrifuged sample extract was made in a test tube with a pipette. The mixture was boiled for 30 min at 100°C then terminated in an ice bath at room temperature. After complete cooling, 1 mL of toluene was added to the mixture and mixed thoroughly, then placed on the bench for 5 min to allow separation of chromophore. The absorbance was read at 520 nm on the UV-visible spectrophotometer (Ultrospec 2100 pro, Amersham Biosciences, Waltham MA, USA) with a 10 mm quartz glass cuvette. From the proline standard curve, the proline concentrations of the C. subternata samples were determined. Proline content was calculated using the formula:
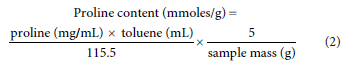
Determination of relative water content (RWC)
An improved version of the method of Sade et al. (2015) was used to determine the RWC of C. subternata leaves. Leaf samples were collected fortnightly at midday (12:00) where 5 top-most leaves per plant were collected, cut into two halves and immediately stored in pre-weighed, labelled glass vials to minimize humidity or vapour loss. The samples were preserved on ice during sampling and quickly transported to the laboratory for RWC determination. Fresh weight (FW) of each sample was determined using a sensitive weighing scale. 2 mL of distilled water was added to each vial, kept in a dark cupboard at room temperature for 4 h to facilitate re-hydration. Thereafter, the turgid leaf samples were removed from the vials and slightly blotted with a paper towel to remove excess water. The blotted leaves were weighed to determine the turgid weight (TW) and later oven-dried at 70°C for 48 h. The dried samples were later weighed to determine the dry weight (DW). Relative water content was calculated using the formula shown below:
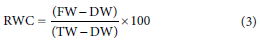
Statistical analysis
Data were analysed with the randomised block factorial ANOVA using SAS statistical software (version 9.4, SAS Institute Inc., Cary, NC, USA, 2013). ANOVA was used for each observation time (harvest/month) separately, as well as with time as subplot factor (Little, 1972). The Shapiro-Wilk test was utilized in testing for deviation from normality (Shapiro, 1965). Fisher's least significant difference was calculated at the 5% level to compare treatment means (Ott, 1998). A probability level of 5% was considered significant for all tests.
RESULTS AND DISCUSSION
Physical and chemical characteristics of the soils
The results of the baseline physicochemical analysis of the soils on which the C. subternata plants were grown are shown in Tables 1 and 2. Stellenbosch granite soil had the highest coarse sand levels (0.5-2 mm) while the lowest was found in Stellenbosch shale. Stellenbosch clovelly had more clay content, with Stellenbosch granite having the lowest (Table 1). The textural classes for Stellenbosch granite, Stellenbosch clovelly and Stellenbosch shale fall within the coarse sandy loam, fine sandy clay loam and sandy clay loam, respectively. Soil pH and other soil nutrients were within the range for normal growth of most plants.
Growth parameters
In general, water stress and soil type had no significant influence (p > 0.05) on plant height, stem diameter or stem circumference throughout the period of the trial (Fig. 1, Table 3). A summary of p-values for separate ANOVAs of growth parameters per month is presented in Table 4.
When compared to the stressed plants, the well-watered (control) treatment had significantly taller plants with greater stem circumference in the first sampling month on Stellenbosch clovelly soil. Thereafter, growth and development of plants did not differ significantly among treatments. The results for the growth parameters of C. subternata in this study contrast with the findings of Tshikhudo et al. (2019) where plant height, stem diameter and the number of leaves of bush tea (Athrixia phylocoides DC). increased with increase in rainfall.
Stress experienced by crops during growth has a cumulative effect, which ultimately reduces the final biomass production (Kamara et al., 2003). This may be the reason why there was generally no significant difference in the growth of C. subternata grown in this trial while the cumulative effects of water stress were only seen in the harvested biomass (Table 5 and Fig. 2). However, a study by Habibi (2018) on Aloe vera demonstrated that short-term water deficit had no significant effect on the leaf biomass. The short duration of the present study may be responsible for the non-significant differences observed in the growth of both the drought-stressed (semi-stressed and stressed) and the well-watered (control) plants.
Yield
The results of the effect of irrigation treatments and soil type on the yield of C. subternata are presented in Fig. 2 and Table 5, respectively. A summarised presentation of p-values for separate ANOVAs on shoot and root biomass is shown in Table 6.
All three irrigation treatments significantly affected the yield (fresh and dry shoots) (p < 0.05). Highest shoot and root yields were recorded in the control treatment on Stellenbosch shale soil, with a progressive yield decline observed with increase in stress level. However, there were no significant differences (p > 0.05) in the root yield of the well-watered (5.05 g) and the semi-stressed (4.33 g) treatments. Stellenbosch clovelly consistently had poor shoot and root yields among the three soil types while there were no significant differences (p > 0.05) between the biomass yields from Stellenbosch granite and Stellenbosch shale.
Eziz et al. (2017) noted that plant growth and biomass production generally decrease with decrease in water availability. However, plants may behave contrary to this, where the cumulative effect of stress during growth may only be visible in the reduced biomass yield (Kamara et al., 2003); which was also observed in this study.
According to Khan et al. (2018), water stress can cause a severe reduction in crop yield, and both the severity and duration of the stress are critical. Water availability is a key factor for sustainable crop production. Its scarcity can have an adverse effect on the physiological and biochemical processes of the plants, thereby causing low yield. The drought-induced yield (root, fresh and dry shoot) decline was comparable to the findings of Zhao et al. (2006), who found that there was a severe reduction in the fresh and dry weights of Brassica napus under water-limiting conditions. Stress at the vegetative stage of plants may lead to reduced stomatal conductance, net photosynthesis and yield (Kerepesi and Galiba, 2000; Fathi and Tari, 2016). The observed yield reduction due to water stress in this study may, therefore, be attributed to impairment of physiological and biochemical processes like photosynthesis, respiration, translocation, ion uptake, and carbohydrate and nutrient metabolism (Ali and Anjum, 2016) during growth. During the period of stress, plants adopt coping mechanisms such as stomatal closure. However, stomatal closure prevents the intake of CO2 into the plant cells, thereby interfering with the Calvin cycle, which will eventually reduce the potential yield of the crop (Ali and Anjum, 2016).
Relative water content (RWC)
RWC is the proportion of water in a leaf, expressed as the percentage of its maximum volumetric water capacity at full turgor (Blum, 2011). It has a direct connection with soil water content and is mostly used as an indicant of water stress in plant leaves. Changes in leaf RWC due to the different irrigation levels in this study are depicted in Fig. 3. At both sampling times, the well-watered treatment consistently had significantly higher (p < 0.05) RWC (87% and 86%, respectively), while the stressed treatment recorded the lowest values (79% and 76% respectively), although there was no significant difference between the semi-stressed (81% and 82%) and the stressed treatments (p > 0.05).
Mabizela (2020) reported similar results, where the stressed and semi-stressed plants had lower RWC compared to the well-watered treatment with the variance showing from the third day after stress initiation. The low RWC in the stressed treatment indicates a stressed plant population compared with the control. Lower RWC in stressed C. subternata leaves used in this study is in accordance with the findings of studies on other species (Arjenaki et al., 2012; Kabbadj et al., 2017). A study on olives supports the outcomes of this research, where the lowest RWC values were reported for severely water-stressed olives (Boussadia et al., 2008). Higher RWC in plant leaves means that the plants had the least water stress, and vice versa.
At the onset of drought, a reduction in stomatal conductance can reduce availability of CO2 for photosynthesis, subsequently leading to inhibition of underlying biochemical processes such as Rubisco carboxylation and electron transport activity, and reducing relative water content and even pigment content (Khalil et al., 2020). The reduction in the leaf RWC due to the strain caused by limited water availability may be attributed to reduction in stomatal conductance after stomatal closure in response to drought stress. As a result of this, there is an observed decrease in the RWC of the stressed C. subternata plants compared to the well-watered plants (Boussadia et al., 2008).
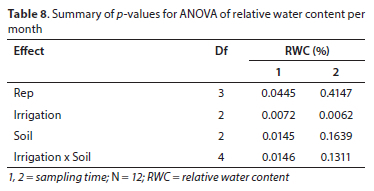
For the three soil types, in the first sampling period, there were no significant differences in the leaf RWC of C. subternata grown on granite and clovelly soil types (p > 0.05). The same observation was made for the comparison between granite- and shale-derived soils. However, water stress significantly decreased (p < 0.05) the relative water content of plants grown in Stellenbosch clovelly when compared with Stellenbosch shale (Table 7). In contrast, the second sampling time showed no significant differences among the treatments. A summary of the p-values for ANOVAs for relative water content per period is presented in Table 8.
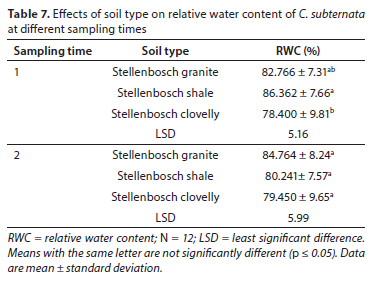
Soil texture is highly influential for water uptake, and may impede root elongation, availability of water, oxygen and nutrients (Khalil et al., 2020). The high percentage of clay in clovelly soil may be responsible for the low leaf RWC in the first sampling period. High clay content in soil increases soil hardness and strength when soil is drying out. As soil strength increases, the more difficult it is for plant roots to access water and nutrients, hence, the lowest RWC in the leaves of C. subternata plants growing on clovelly soil. However, in the second sampling period, since this was a pot experiment, the packaging of the soil might have altered the actual field structure, allowing more macropores in the soils with high clay content than is likely to exist in the field (Khalil et al., 2020). The presence of these macropores may have contributed to the non-significant effects observed among all treatments in response to the water treatment.
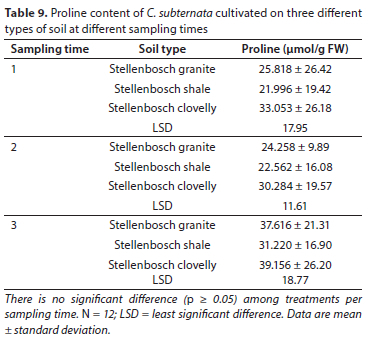
Proline
Several abiotic factors, such as water stress, high temperatures and salinity, can cause protein modification, membrane injury and osmotic stress in plants (Meena et al., 2019). Plants respond to water stress by building-up osmolytes such as proline, glycine betaine, glycerol and many more, in order to minimize and tolerate cell injury (Sharma et al., 2019). Figure 4 shows that the stressed C. subternata plants in this study consistently had significantly higher proline contents in all sampling periods, while the lowest proline content was found in the well-watered (p < 0.05) plants. Significantly higher proline content was observed in the stressed plants compared to the other two treatments in the first sampling period. However, no significant difference was observed among all treatments in the second and third sampling periods (p > 0.05).
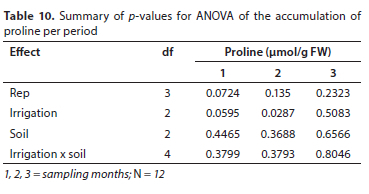
The obtained results are comparable to those reported by Mabizela (2020) on proline contents of C. subternata, where proline concentration increased massively in stressed treatments, increased slightly in semi-stressed plants, and was constant in control treatments. Higher proline content were also reported in wheat, Amaranthus species and Achillea species after being subjected to water stress (Keyvan, 2010; Slabbert and Krüger, 2014; Gharibi et al., 2016). Low proline content in plants indicates minimum water stress, and vice-versa. The high and significant levels of proline that were observed between treatments during the third sampling period may be attributed to the plants having reached the reproductive stage and having started flowering. Proline can accumulate in plants under both stress and non-stress conditions, although it is produced at low levels in all tissues in unstressed conditions (Kavi Kishor et al., 2015). As a metabolite and signal molecule, proline plays a crucial role in the synthesis of protein and the response of plant cells to environmental stresses (Mattioli et al., 2009). Proline levels may increase during wounding and pathogen attack in some tissues, different stages of plant growth and development, nodule formation, fertilization, cytokinesis, apoptosis, senescence, and cell wall lignification. Under normal physiological (un-stressed) conditions, plants accumulate high amounts of proline during the transition to flower initiation (Kavi Kishor et al., 2015), thus suggesting that proline may have a role to play in flower initiation and its subsequent development. Soil type did not have any significant effect (p > 0.05) on the proline contents of the plants (Table 9). A summary of p-values for ANOVAs of the accumulation of proline per month is presented in Table 10.
CONCLUSION
From this study, it is evident that different deficit irrigation levels and soil type had no significant effects on growth parameters of C. subternata. Likewise, soil type had no impact on the proline, RWC and the yield of the plants. Water stress increased the proline content, resulting in lower RWC. However, deficit irrigation had a significant effect on the yield (root, fresh and dry shoot biomass). The higher the water stress, the lower the shoot and root biomass yield and vice-versa. Although, the well-watered and the semi-stressed plants gave higher shoot yield, more research is still needed to determine the tea quality of the stressed and unstressed C. subternata plants.
ACKNOWLEDGMENTS
The Department of Science and Innovation (DSI) of South Africa for funding the project; the Department of Higher Learning and Training for supporting the study financially through Nurturing Emerging Scholars Programme (NESP); the staff of Soil Science division, ARC Infruitec-Nietvoorbij for technical support and providing their facilities; Dr M van der Rijst for assistance with statistical analysis.
CONTRIBUTION OF AUTHORS
Mary-Jane Seji Mahlare - data collection, sample analysis, writing the initial draft, writing revision; Dr Muinat N Lewu -conceptualization, methodology, validation, student supervision, writing revision, project leadership, project management; Prof Francis B Lewu - conceptualization, methodology, validation, writing revision, student supervision; Dr Cecilia Bester -conceptualization, methodology, project leadership, project management, funding acquisition.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
ÁBRAHÁM E, HOURTON-CABASSA C, ERDEI L and SZABADOS L (2010) Methods for determination of proline in plants. In: Plant stress tolerance. Humana Press, New Jersey. 317-331. https://doi.org/10.1007/978-1-60761-702-0_20 [ Links ]
ALI N and ANJUM MM (2016) Drought stress: major cause of low yield and productivity. Austin Environ. Sci. 1 10-12. [ Links ]
ARJENAKI FG, JABBARI R and MORSHEDI A (2012) Evaluation of drought stress on relative water content, chlorophyll content and mineral elements of wheat (Triticum aestivum L.) varieties. Int. J. Agric. Crop Sci. 4 (11) 726-729. [ Links ]
BLUM A (2011) Plant water relations, plant stress and plant production. In: Plant Breeding For Water-Limited Environments. Springer, New York. 11-52. https://doi.org/10.1007/978-1-4419-7491-4_2 [ Links ]
BOUSSADIA O, MARIEM FB, MECHRI B, BOUSSETTA W, BRAHAM M and EL HADJ SB (2008) Response to drought of two olive tree cultivars (cv Koroneki and Meski). Sci. Hortic. 116 (4) 388-393. https://doi.org/10.1016/j.scienta.2008.02.016 [ Links ]
CEREKOVIC N, PAGTER M, KRISTENSEN HL, PEDERSEN HL, BRENNAN R and PETERSEN KK (2013) Effects of drought stress during flowering of two pot-grown blackcurrant (Ribes nigrum L.) cultivars. Sci. Hortic. 162 365-373. https://doi.org/10.1016/j.scienta.2013.08.026 [ Links ]
CHAKHCHAR A, LAMAOUI M, WAHBI S, FERRADOUS A, EL MOUSADIK A, IBNSOUDA-KORAICHI S, FILALI-MALTOUF A and EL MODAFAR C (2015) Leaf water status, osmoregulation and secondary metabolism as a model for depicting drought tolerance in Argania spinosa. Acta Physiol. Plant. 37 (4) 1-16. https://doi.org/10.1007/s11738-015-1833-8 [ Links ]
CONNOR R (2015) The United Nations World Water Development Report 2015: Water for a Sustainable World, Vol. 1. UNESCO Publishing, Paris. [ Links ]
DU TOIT J, JOUBERT E and BRITZ TJ (1998) Honeybush tea - a rediscovered indigenous South African herbal tea. J. Sustain. Agric. 12 (2-3) 67-84. https://doi.org/10.1300/J064v12n02_06 [ Links ]
EZIZ A, YAN Z, TIAN D, HAN W, TANG Z and FANG J (2017) Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 7 (24) 11002-11010. https://doi.org/10.1002/ece3.3630 [ Links ]
FATHI A and TARI DB (2016) Effect of drought stress and its mechanism in plants. Int. J. Life Sci. 10 (1) 1-6. [ Links ]
GHARIBI S, TABATABAEI BES, SAEIDI G and GOLI SAH (2016) Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of Achillea species. Appl. Biochem. Biotechnol. 178 (4) 796-809. https://doi.org/10.1007/s12010-015-1909-3 [ Links ]
HABIBI G (2018) Effects of mild and severe drought stress on the biomass, phenolic compounds production and photochemical activity of Aloe vera (L.) Burm. f. Acta. Agric. Slov. 111 (2) 463-476. https://doi.org/10.14720/aas.2018.111.2.19 [ Links ]
HARB A, KRISHNAN A, AMBAVARAM MM and PEREIRA A (2010) Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 154 (3) 1254-1271. https://doi.org/10.1104/pp.110.161752 [ Links ]
HORN A (2019) Honey bush tea - small industry, big potential. Farmer's Weekly 5 April 2019. 36-38. [ Links ]
JONES HG (2007) Monitoring plant and soil water status: established and novel methods revisited and their relevance to studies of drought tolerance. J. Exp. Bot. 58 (2) 119-130. https://doi.org/10.1093/jxb/erl118 [ Links ]
JOUBERT E, GELDERBLOM WCA, LOUW A and DE BEER D (2008) South African herbal teas: Aspalathus linearis, Cyclopia spp. and Athrixiaphylicoides-A review. J. Ethnopharmacol. 119 (3) 376-412. https://doi.org/10.1016/j.jep.2008.06.014 [ Links ]
JOUBERT E, JOUBERT ME, BESTER C, DE BEER D and DE LANGE JH (2011) Honeybush (Cyclopia spp.): From local cottage industry to global markets - the catalytic and supporting role of research. S. Afr. J. Bot. 77 (4) 887-907. https://doi.org/10.1016/j.sajb.2011.05.014 [ Links ]
JOUBERT EDBD and DE BEER D (2011) Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. S. Afr. J. Bot. 77 (4) 869-886. https://doi.org/10.1016/j.sajb.2011.07.004 [ Links ]
KABBADJ A, MAKOUDI B, MOURADI M, PAULY N, FRENDO P and GHOULAM C (2017) Physiological and biochemical responses involved in water deficit tolerance of nitrogen-fixing Vicia faba. PloS One 12 (12) p.e0190284. https://doi.org/10.1371/journal.pone.0190284 [ Links ]
KAMARA AY, MENKIR A, BADU-APRAKU B and IBIKUNLE O (2003) The influence of drought stress on growth, yield, and yield components of selected maize genotypes. J. Agri. Sci. 141 (1) 43-50. https://doi.org/10.1017/s0021859603003423 [ Links ]
KARDILE PB, DAHATONDE KN, RAKSHE MV and BURONDKAR MM (2018) Effect of moisture stress on leaf relative water content (RWC) of four cowpea (Vigna unguiculata L. walp.) genotypes at different stages of growth. Int. J. Curr. Microbiol. Appl. Sci. 7 2645-2649. https://doi.org/10.20546/ijcmas.2018.704.301 [ Links ]
KAVI KISHOR PB, HIMA KUMARI P, SUNITA MSL and SREENIVASULU N (2015) Role of proline in cell wall synthesis and plant development and its implications in plant ontogeny. Front. Plant Sci. 6 544 pp. https://doi.org/10.3389/fpls.2015.00544 [ Links ]
KEREPESI I and GALIBA G (2000) Osmotic and salt stress-induced alteration in soluble carbohydrate content in wheat seedlings. Crop Sci. 40 (2) 482-487. https://doi.org/10.2135/cropsci2000.402482x [ Links ]
KEYVAN S (2010) The effects of drought stress on yield, relative water content, proline, soluble carbohydrates and chlorophyll of bread wheat cultivars. J. Anim. Plant Sci. 8 (3) 1051-1060. [ Links ]
KHALIL AM, MURCHIE EH and MOONEY SJ (2020) Quantifying the influence of water deficit on root and shoot growth in wheat using x-ray computed tomography. AoB Plants. 12 (5) 36 pp. https://doi.org/10.1093/aobpla/plaa036 [ Links ]
KHAN A, PAN X, NAJEEB U, TAN DKY, FAHAD S, ZAHOOR R and LUO H (2018) Coping with drought: stress and adaptive mechanisms, and management through cultural and molecular alternatives in cotton as vital constituents for plant stress resilience and fitness. Biol. Res. 51. https://doi.org/10.1186/s40659-018-0198-z. [ Links ]
LI-PING BAI, FANG-GONG SUI, TI-DA GE, ZHAO-HUI SUN, YIN-YAN LU and GUANG-SHENG ZHOU (2006) Effect of soil drought stress on leaf water status, membrane permeability and enzymatic antioxidant system of maize. Pedosphere. 16 (3) 326-332. https://doi.org/10.1016/S1002-0160(06)60059-3 [ Links ]
LITTLE TM and HILLS FJ (1972) Statistical Methods in Agricultural Experimentation. University of California, Davis. [ Links ]
LONG K and PIJANOWSKI BC (2017) Is there a relationship between water scarcity and water use efficiency in China? A national decadal assessment across spatial scales. Land Use Policy 69 502-511. https://doi.org/10.1016/j.landusepol.2017.09.055 [ Links ]
LUGOJAN C and CIULCA S (2011) Evaluation of relative water content in winter wheat. J. Hortic. For. Biotechnol. 15 (2) 173-177. [ Links ]
MABIZELA GS (2020) Metabolic and quality profiling of Cyclopia subternata and C. genistoides in response to seasonal variation and drought stress. PhD thesis, Tshwane University of Technology. [ Links ]
MATTIOLI R, FALASCA G, SABATINI S, ALTAMURA MM, COSTANTINO P and TROVATO M (2009) The proline biosynthetic genes P5CS1 and P5CS2 play overlapping roles in Arabidopsis flower transition but not in embryo development. Physiol. Plant. 137 (1) 72-85. https://doi.org/10.1111/j.1399-3054.2009.01261.x [ Links ]
MCGAW LJ, STEENKAMP V and ELOFF JN (2007) Evaluation of Athrixia bush tea for cytotoxicity, antioxidant activity, caffeine content and presence of pyrrolizidine alkaloids. J. Ethnopharmacol. 110 (1) 16-22. https://doi.org/10.1016/j.jep.2006.08.029 [ Links ]
MEENA M, DIVYANSHU K, KUMAR S, SWAPNIL P, ZEHRA A, SHUKLA V, YADAV M and UPADHYAY RS (2019) Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 5 (12) p.e02952. https://doi.org/10.1016/j.heliyon.2019.e02952 [ Links ]
OBIDIEGWU JE, BRYAN GJ, JONES HG and PRASHAR A (2015) Coping with drought: stress and adaptive responses in potato and perspectives for improvement. Front. Plant Sci. 6. https://doi.org/10.3389/fpls.2015.00542 [ Links ]
OTT RL and LONGNECKER MT (2015) An Introduction to Statistical Methods and Data Analysis. Cengage Learning, Boston. [ Links ]
PER TS, KHAN NA, REDDY PS, MASOOD A, HASANUZZAMAN M, KHAN MIR and ANJUM NA (2017) Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant. Physiol. Biochem. 115 126-140. https://doi.org/10.1016/j.plaphy.2017.03.018 [ Links ]
RAMPEDI I and OLIVIER J (2005) The use and potential commercial development of Athrixiaphylicoides. Acta. Acad. 37 (3) 165-183. [ Links ]
RSA (Republic of South Africa) (2015) No. 572. Limiting the use of water for irrigation, domestic and urban purposes from Goedertrouw, Hazelmere, Klipfontein, Hluhluwe, Lake Merthley, Umzinto, EJ Smith and Buffalo Systems in KZN. Government Gazette No. 38924, 03 July 2015. Government Printer, Pretoria. [ Links ]
SADE N, GALKIN E and MOSHELION M (2015) Measuring Arabidopsis, tomato and barley leaf relative water content (RWC). Biol. Protoc. 5 (8) 1451-1451. https://doi.org/10.21769/bioprotoc.1451 [ Links ]
SAS INSTITUTE, INC. (2013) Statistical Analysis Software. Users' Guide Statistics Version 9.4. SAS Institute Inc., Cary, North Carolina. [ Links ]
SHAPIRO SS and WILK MB (1965) An analysis of variance test for normality (complete samples). Biometrika. 52 (3/4) 591-611. https://doi.org/10.1093/biomet/52.3-4.591 [ Links ]
SHARMA HS, FENG L, MURESANU DF, CASTELLANI RJ and SHARMA A (2019) Neuroprotective effects of a potent bradykinin B2 receptor antagonist HOE-140 on microvascular permeability, blood flow disturbances, edema formation, cell injury and nitric oxide synthase upregulation following trauma to the spinal cord. Academic Press. Int. Rev. Neurobiol. 146 103-152. https://doi.org/10.1016/bs.irn.2019.06.008 [ Links ]
SIDDIQUE A and DUBEY AP (2017) Phyto-toxic effect of heavy metal (CdCl2) on seed germination, seedling growth and antioxidant defence metabolism in wheat (Triticum aestivum L.) variety HUW-234. Int. J. Bio-resour. 8 (2) 261-267. https://doi.org/10.23910/ijbsm/2017.8.2.1684 [ Links ]
SLABBERT MM and KRÜGER GHJ (2014) Antioxidant enzyme activity, proline accumulation, leaf area and cell membrane stability in water stressed Amaranthus leaves. S. Afr. J. Bot. 95 123-128. https://doi.org/10.1016/j.sajb.2014.08.008 [ Links ]
SOLTYS-KALINA D, PLICH J, STRZELCZYK-ZYTA D, SLIWKA J and MARCZEWSKI W (2016) The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of 'Katahdin'-derived potato cultivars. Breed. Sci. 66 (2) 328-331. https://doi.org/10.1270/jsbbs.66.328 [ Links ]
SONI RP, KATOCH M, KUMAR A, LADOHIYA R and VERMA P (2015) Tea: production, composition, consumption and its potential as an antioxidant and antimicrobial agent. Int. J. Food. Ferment. Technol. 5 (2) 95-106. https://doi.org/10.5958/2277-9396.2016.00002.7 [ Links ]
SURENDAR KK, DEVI DD, RAVI I, JEYAKUMAR P and VELAYUDHAM K (2013) Water stress affects plant relative water content, soluble protein, total chlorophyll content and yield of ratoon banana. Int. J. Hortic. 3 (17) 96-103. [ Links ]
TSHIKHUDO PP, NTUSHELO K, KANU SA and MUDAU FN (2019) Growth response of bush tea (Athrixia phylocoides DC) to climatic conditions in Limpopo Province, South Africa. S. Afr. J. Bot. 121 500-504. https://doi.org/10.1016/j.sajb.2018.12.012 [ Links ]
VAN WYK BE and GERICKE N (2000) People's Plants: A Guide to Useful Plants of Southern Africa. Briza Publications, Pretoria. [ Links ]
VELASCO-MUNOZ JF, AZNAR-SÁNCHEZ JA, BELMONTE-URENA LJ and LÓPEZ-SERRANO MJ (2018) Advances in water use efficiency in agriculture: A bibliometric analysis. Water 10 (4) 377. https://doi.org/10.3390/w10040377. [ Links ]
WWAP (United Nations World Water Assessment Programme) (2012) The United Nations World Water Development Report 4: Managing Water under Uncertainty and Risk. UNESCO, Paris. https://doi.org/10.18356/cda9571e-en [ Links ]
WWF (2018) Agricultural Water File: Farming for a drier future. http://awsassets.wwf.org.za/downloads/wwfwaterfiles19july2018.pdf (Accessed 16 November 2022). [ Links ]
ZHAO TJ, SUN S, LIU Y, LIU JM, LIU Q, YAN YB and ZHOU HM (2006) Regulating the drought-responsive element (DRE)-mediated signaling pathway by synergic functions of trans-active and trans-inactive DRE binding factors in Brassica napus. J. Biol. Chem. 281 (16) 10752-10759. https://doi.org/10.1074/jbc.m510535200 [ Links ]
 Correspondence:
Correspondence:
FB Lewu
Email: LewuF@cput.ac.za
Received: 9 March 2022
Accepted: 4 January 2023














